-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(9): 2407-2410
doi:10.5923/j.ajmms.20241409.60
Received: Aug. 26, 2024; Accepted: Sep. 23, 2024; Published: Sep. 30, 2024

Microbiocenosis of the Neovagina and Intestines in the Postoperative Period
Negmadzhanov Bakhodur Boltaevich, Akhmedov Zarif Shamsidinovich, Ganiev Fakhriddin Istamkulovich
Samarkand State Medical Institute, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Today, the number of patients requiring colpopoiesis surgery is increasing significantly. In the postoperative period, much attention is paid to the problem of vaginal microflora and is an urgent topic that plays an important role in assessing a woman's reproductive health. There is a wide variety of data on the microflora of the artificial vagina, which varies depending on the stage of the rehabilitation period after surgery for colpopoiesis, described by a small number of authors, which does not allow standardizing these indicators. This article presents data on the microbial flora of the sigmoid neovagina.
Keywords: Sigmoid neovagina, Colpopoiesis, Microbiology, Bacterial flora
Cite this paper: Negmadzhanov Bakhodur Boltaevich, Akhmedov Zarif Shamsidinovich, Ganiev Fakhriddin Istamkulovich, Microbiocenosis of the Neovagina and Intestines in the Postoperative Period, American Journal of Medicine and Medical Sciences, Vol. 14 No. 9, 2024, pp. 2407-2410. doi: 10.5923/j.ajmms.20241409.60.
Article Outline
1. Introduction
- One of the most significant problems of modern health care around the world is the problem of reproductive and sexual health. Anomalies of the uterus and vagina are congenital defects that have become increasingly common in recent years. Mayer-Rokitansky-Küster-Hauser syndrome (MRKHS) is a leading cause of vaginal and uterine aplasia with an incidence of 1 in 4500 female births. In recent decades, a number of scientific studies have been carried out all over the world to develop the most evidence-based treatment methods and identify risk factors for abnormal development of the genital organs in girls, such as uterine and vaginal defects (Negmadzhanov B.B., 2021; Kirpatovsky I.D., Ugryumova L. Yu, Uvarova E. V., 2017). Treatment of Rokitansky-Küstner syndrome is surgical and comes down to the creation of a neovagina [3,4,8,9]. To create a neovagina, sigmoid colpopoiesis is successfully used (Negmadjanov B.B. et al., 2020).After sigmoidal colpopoiesis, pathogens of bacterial infections often develop and persist for a long time, causing colpitis, bacterial vaginosis, trophic changes in neovaginal tissue, which requires complex therapy, taking into account the microbial factor and the severity of the inflammatory process (Navruzov S. N., Navruzov B. S., Shaimardanov E.K., 2017). It is the saturation of the vaginal wall with lactobacilli in the postoperative period that makes it possible to change the qualitative composition of the microflora, thereby having a beneficial effect on the course of the postoperative period, affecting the outcomes of surgical treatment [2,5,6]. There are reports of high therapeutic and prophylactic effectiveness of probiotics in inflammatory processes of various localizations, including purulent-inflammatory complications after surgery (Andreeva I.V., 2015). The study of vaginal microbiocenosis after sigmaid colpopoiesis and the improvement of methods of clinical and laboratory diagnosis of this disease will contribute to the understanding of the mechanisms of the pathogenesis of inflammatory diseases, complicating its course and more effective tactics for managing patients. This pathology, while remaining stable and not threatening the patient’s life, causes severe physical and moral suffering due to the emergence of a characteristic “symptom complex”, in which developing complications play an important role, with the ensuing adverse consequences [1,7,8].
2. Purpose of the Study
- To develop preventive measures to reduce the incidence of postoperative infectious and inflammatory complications.
3. Material and Research Methods
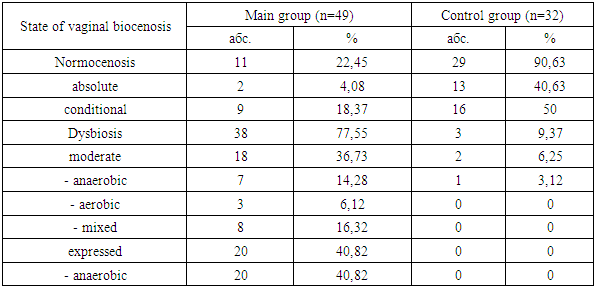
- The study included 81 patients after undergoing sigmaid colpopoiesis, who were divided into 2 groups: the main group - 49 patients with complaints of heavy discharge from the genital tract and the control group - 32 women without clinical signs of bacterial vaginosis.
4. Results of the Study
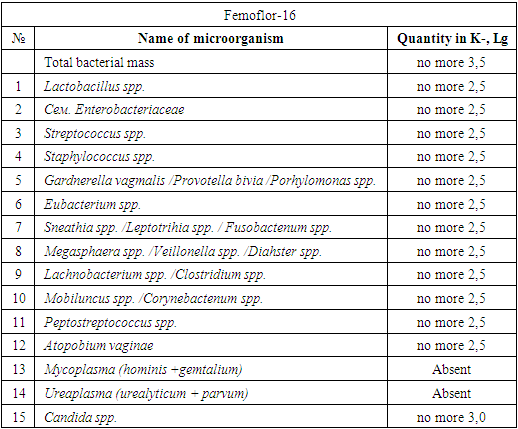
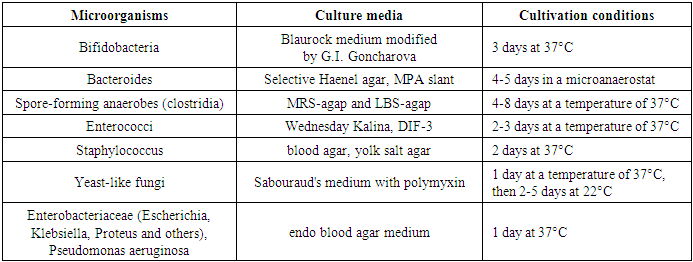
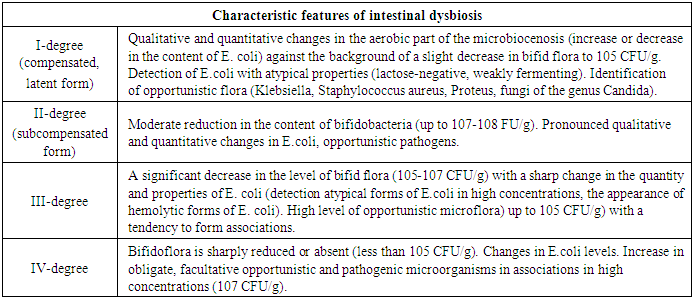
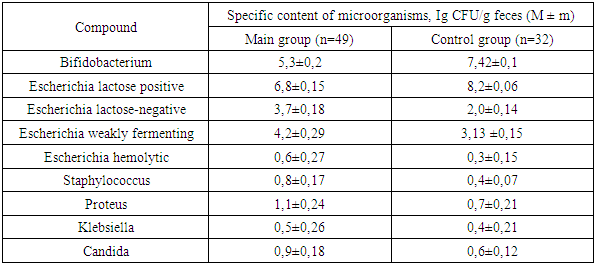
- When studying the anamnesis of the women who took part, special attention was paid to such parameters as: age, social status and material and living conditions, education, characteristics of professional activity, occupational hazards; diseases suffered in different years of life, their course, outcome; the presence of diseases that are a manifestation of connective tissue failure (varicose veins, hernias of various locations, a history of frequent joint dislocations); the presence of diseases accompanied by increased intra-abdominal pressure (cough, constipation); complaints made by a woman upon admission to hospital, main symptoms.The study of vaginal microbiocenosis was carried out using complex quantitative real-time PCR using Femoflor-16 reagents in a detection amplifier in 81 patients. The Femoflor-16 reagent kit is intended for the detection of DNA of opportunistic microorganisms, lactobacilli and human genomic DNA (as a control for taking biological material).Quantitative assessment of the urogenital biota was carried out in absolute and relative terms (Table 1). To quantify the normal flora and UPM, relative indicators were used, which were calculated as the difference in logarithms to base 10, using the formula: log10(-) = log10x - log10y. The relative indicator of normal flora was the difference in logarithms obtained for the total bacterial mass and normal flora. The test determines the total concentration of bacterial DNA - total bacterial mass (TBM) - and the concentration (absolute and relative) of the following types/genera of microorganisms: Lactobacillus, Enterobacteriaceae, Streptococcus, Staphylococcus, Gardnerella vaginalis/ Prevotella bivia/ Porphyromonas, Eubacterium, Sneathia/ Leptotrichia/ Fusobacterium, Megasphaera/ Veillonella/ Dialister, Lachnobacterium/ Clostridium, Corynebacterium/ Mobiluncus, Peptostreptococcus, Atopobium vaginae. The ratio of these bacteria determines the state of the vaginal microbiocenosis - normocenosis or dysbiosis. Dysbiosis, in turn, is assessed by the degree of severity (moderate or severe dysbiosis) and the predominance of aerobic or anaerobic conditionally pathogenic microflora (aerobic or anaerobic dysbiosis, respectively). A study of intestinal microflora was carried out on 81 patients in accordance with the methodological recommendations of N.M. Grachevoy et al., (1986) [Gracheva N.M., 1986; Znamensky V.A. et al., 1986; Raevsky K.K. et al, 1997; Dobrynin V.M. et al., 1999; Efimov B.A. et al., 2002, etc.;]. The state of intestinal microbiocenosis was assessed in all patients upon presentation. Determination of the species of bacteria was carried out using generally accepted methods and identification schemes (Table 2). Microbial colonization of the intestine was assessed by the frequency of isolation of symbionts and by intensity - CFU/g, the tenth logarithm of the average number of microorganisms (lg CFU) isolated from the test material. Assessment of dysbacterial abnormalities in the intestines according to the degree of dysbiosis was carried out according to the classification of Kuvaev I.B., Ladodo K.S. (1991) [Kuvaeva I.B., Ladodo K.S. Microecological and immune disorders in children. M.: Medicine; 1991; p.240].
|
|
|
|
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML