-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(9): 2393-2396
doi:10.5923/j.ajmms.20241409.57
Received: Aug. 23, 2024; Accepted: Sep. 16, 2024; Published: Sep. 30, 2024

Analysis of the Effectiveness of the Immune Reaction When Using Vaccination Against the Hepatitis B Virus
B. M. Tadjiev, M. B. Matyakubov, D. M. Urunova
Republican Specialized Scientific and Practical Medical Center for Epidemiology, Microbiology, Infectious and Parasitic Diseases, Tashkent, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

It is known that the main way to prevent viral hepatitis B is vaccination. The goal is to evaluate the effectiveness of the immune response against the background of immunization against the hepatitis B virus. Materials and methods. An enzyme immunoassay was carried out on 280 blood samples of vaccinated children for the presence of HBs antigen (rapid test and ELISA) and antibodies against HBs antigen (using an enzyme immunoassay). To determine the HBsAg express method, the American-made Aria test system “HBs COMBO RAPID TEST” (CTK Biotech, Inc, expiration date 2025-01-29) was used. After confirming the express test, an ELISA test was performed to check the antigen using the Vector-best test system “Vectogep B - HBs-antigen”. The amount of immune Anti-HB antibodies against hepatitis B vaccination was determined by ELISA using the “Vector Best” test system (international certificate ISO 13485, expiration date 2024-05-12) “VectoHBsAg-antibody”. Results and conclusions. The study did not reveal positive results for the hepatitis B viral antigen, which is proof of the effectiveness of immunity acquired as a result of vaccination. It is important to note that over time, this immunity may begin to wane due to lack of exposure to the antigen. This emphasizes the need for regular monitoring of the condition and, if necessary, additional vaccinations in order to maintain the body's protective functions.
Keywords: Viral hepatitis B, Gender differences, Immunity, Vaccine
Cite this paper: B. M. Tadjiev, M. B. Matyakubov, D. M. Urunova, Analysis of the Effectiveness of the Immune Reaction When Using Vaccination Against the Hepatitis B Virus, American Journal of Medicine and Medical Sciences, Vol. 14 No. 9, 2024, pp. 2393-2396. doi: 10.5923/j.ajmms.20241409.57.
Article Outline
1. Introduction
- Viral hepatitis is one of the global health problems, causing great damage to human health and healthcare systems [1].According to the World Health Organization (WHO), 820,000 people die from hepatitis B every year, of whom more than 650,000 died from liver cirrhosis or hepatocellular carcinoma and about 130,000 from acute viral hepatitis. [2.8]. Currently, 296 million people worldwide are infected with chronic hepatitis B, and an additional 1.5 million people become ill with the disease each year. Hepatocellular carcinoma ranks third in mortality among cancers in the world [3].Hepatitis B virus can be effectively prevented through vaccination. The introduction of the vaccine into national immunization programs has led to a reduction in transmission of the virus in highly endemic countries [6]. To increase adherence to a healthy lifestyle and vaccination, it is important to carry out outreach to the population and introduce educational programs [5]. This will help prevent infection and the development of chronic forms of the disease [4]. By 2030, vaccination is expected to prevent approximately 210 million cases of chronic hepatitis B virus infection and eliminate approximately 1.1 million deaths [7].
2. Material and Research Methods
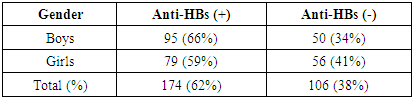
- The study was conducted at the Republican Specialized Center for Epidemiology, Microbiology, Infectious and Parasitic Diseases in the city of Tashkent. Blood samples were taken from 280 vaccinated children against viral hepatitis B. For analysis, an express method was used (Aria HBsAg Kombo Rapid test, manufactured by CTK Biotech, expiration date until January 29, 2025) to determine the presence of HBsAg and an ELISA test system to assess immunological tension (Vecto HBsAg antibody test system, certified according to ISO 13485, valid until 05/12/2024). Blood samples from patients treated for hepatitis B virus in the clinic were also used as a control group, and their sera were analyzed using a rapid test for HBsAg and ELISA tests for the quantitative determination of antibodies to HBsAg. After collection, the blood was transported to the laboratory with a cold reagent, blood serum samples were stored in a refrigerator at -200C, and before use were kept at room temperature for 1 hour, then tests were carried out. Among the children examined, boys made up 52%, girls 48%. Analysis of the age of the examined children showed that children under 1 year old accounted for 9% (24), 1-3 years old - 36% (101), 3-5 years old - 30% (84), 5-8 years old - 25% (71). Of the 280 children who participated in the study, vaccination cards for 259 children were analyzed.Statistical analysis was carried out using the StatTech v program. 4.1.2 (developer - Stattekh LLC, Russia).
3. Results and Discussion
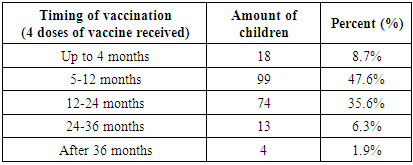
- As a result of the analysis of vaccination cards, it was found that out of 280 children, 7.5% (21) had no vaccination cards. Among the examined children who had vaccination cards, 5.4% (15) received one dose of the vaccine, 2.5% (7) received 2 doses, 8.9% (25) received 3 doses, 75.7% of children (212) received 4 doses of the vaccine. (Fig. 1).
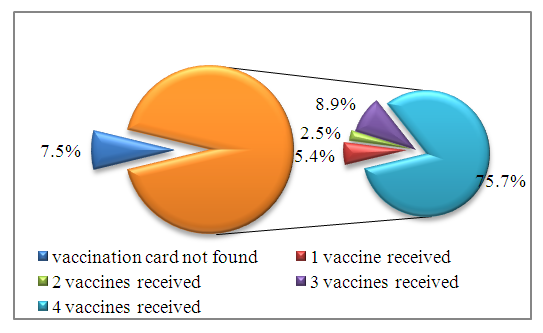 | Figure 1. Distribution of examined children depending on the frequency of vaccination. (n=280) |
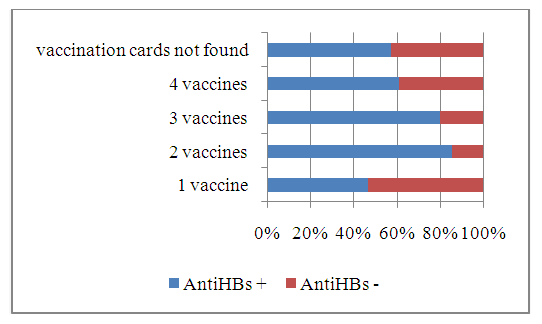 | Figure 2. Proportion of children vaccinated against HBV with antibodies to HBsAg |
|
|
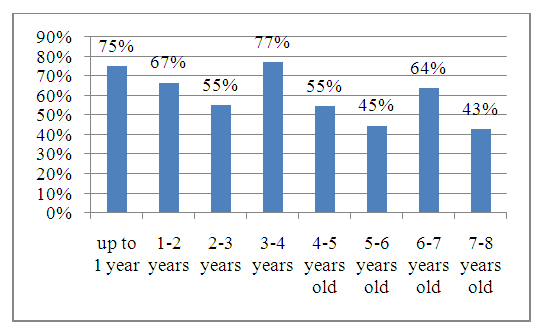 | Figure 3. The proportion of children with antibodies to HBsAg depending on the age of the children |
|
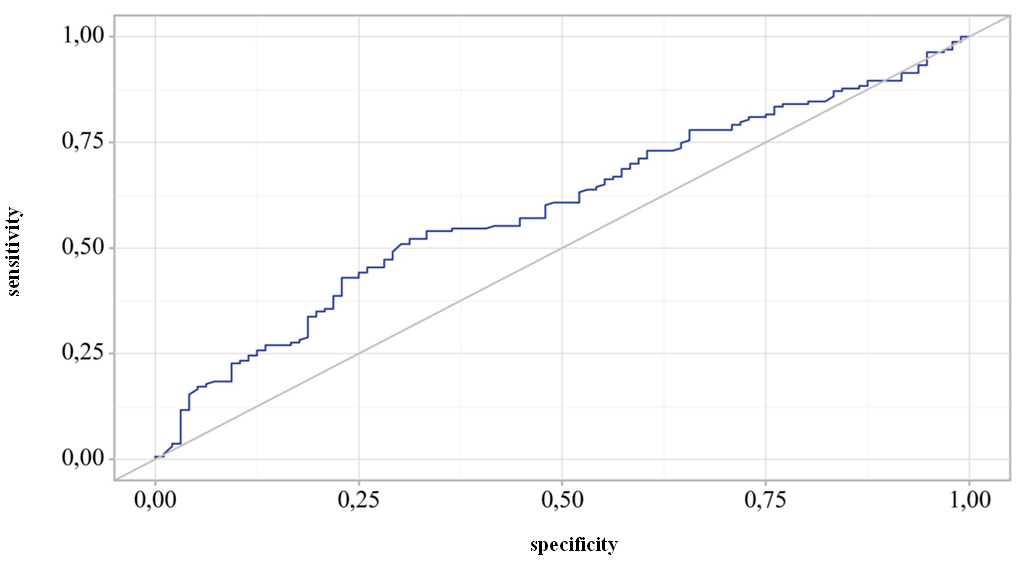 | Figure 4. ROC curve representing the dependence of the probability of detecting antibodies to HBsAg on the length of time since the last vaccination |
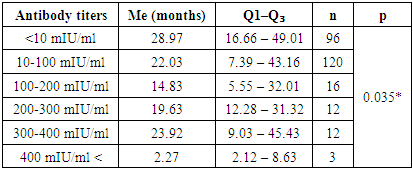
4. Conclusions
- 1. According to the national immunization schedule, 8.7% of children received the full dose of hepatitis B vaccine on time, and 56% of children received it within one year.2. A study related to the detection of HBs antibodies depending on the age of children showed that the percentage of antibody detection decreases with age. Antibodies were detected in 75% of children under the age of one year, and by 7 years this figure was 43%.3. The concentration of HBs antibodies in the blood did not exceed 10 mIU/ml in 37.5% (105) children, 10-100 mIU/ml in 46.8% (131), and in 15.7% (44) children more than high titers (more than 100 mIU/ml).4. ROC analysis showed that the probability of detecting HBsAg antibodies after the last vaccination persists for up to 21,367 months.5. The concentration of antibodies to HBsAg in the blood serum of children decreases over time after vaccination.Thus, the study did not find positive results for hepatitis B virus antigen, confirming the effectiveness of the immunity obtained as a result of vaccination. It is important to note that this immunity may wane over time due to lack of exposure to the antigen. Therefore, regular monitoring of the condition and, if necessary, additional vaccination will help maintain the body's protective functions. Immunity gained through vaccination is an important element of protection against viruses and diseases. However, like any system, it requires care and attention. Regular observation and monitoring of the state of immunity will help to promptly identify possible problems and take the necessary measures to eliminate them.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML