-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(9): 2388-2392
doi:10.5923/j.ajmms.20241409.56
Received: Aug. 25, 2024; Accepted: Sep. 17, 2024; Published: Sep. 30, 2024

Adaptive Mechanisms and Correction of the Immune System During Coronavirus Infection Caused by SARS-CoV-2
Abilov P. M.1, Iriskulov B. U.2, Boboeva Z. N.3, Saydalikhodjaeva O. Z.4, Azimova S. B.5
1Assistant, PhD, Department of Normal and Pathological Physiology, Tashkent Medical Academy, Uzbekistan
2Prof., DSc, Head of the Department of Normal and Pathological Physiology, Tashkent Medical Academy, Uzbekistan
3PhD, Senior Lecturer, Department of Normal and Pathological Physiology, Tashkent Medical Academy, Uzbekistan
4PhD, Assistant Professor of the Department of Normal and Pathological Physiology, Tashkent Medical Academy, Uzbekistan
5DSc, Professor of the Department of Normal and Pathological Physiology TMA, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This article analyzes the effectiveness of a new drug based on G. Lucidum and Alkhadai in the treatment of coronavirus infection caused by COVID-19. The pathophysiological mechanism of the influence of a new drug on the course of coronavirus infection is given.
Keywords: Pathophysiological mechanisms, Coronavirus infection, G. lucidum, Alkhadaya, Acute respiratory syndrome
Cite this paper: Abilov P. M., Iriskulov B. U., Boboeva Z. N., Saydalikhodjaeva O. Z., Azimova S. B., Adaptive Mechanisms and Correction of the Immune System During Coronavirus Infection Caused by SARS-CoV-2, American Journal of Medicine and Medical Sciences, Vol. 14 No. 9, 2024, pp. 2388-2392. doi: 10.5923/j.ajmms.20241409.56.
Article Outline
1. Introduction
- The severe acute respiratory syndrome coronavirus (COVID-19) pandemic caused by coronarus-2 (SARS-CoV-2) has caused nearly 270 million confirmed cases and more than 5.2 million deaths worldwide. Only on December 13-19, 2021, 4.1 million cases and slightly less than 45,000 new deaths were detected [4].To date, the causative agent of the coronavirus infection COVID-19 is SARS-CoV-2 with single-stranded RNA. Based on accumulated research on the pathogenesis of coronavirus, several molecular targets have been identified, such as 3-chymotrypsin-like cysteine protease (3CLpro), papain-like protease (PLpro), and RNA-dependent RNA polymerase (RDRP) [2].The development of COVID-19 is associated with acute inflammation and an immune response that can trigger a hyperinflammatory syndrome called “cytokine storm” [7].The pathogenetic mechanism of the "cytokine storm" is due to the excessive release of pro-inflammatory cytokines such as IL-1, IL-6, TNF-α.In a study by Angélica Jayk Bernal et al. (2021) investigated molnupiravir, which is a small molecule ribonucleoside prodrug of N-hydroxycytidine (NHC) that has activity against SARS-CoV-2 and other RNA viruses and is a high barrier to the development of resistance. Following oral administration of molnupiravir, NHC circulates systemically and is phosphorylated intracellularly to NHC triphosphate [9]. NHC triphosphate is incorporated into viral RNA by the viral RNA polymerase and subsequently misdirects the viral polymerase to incorporate guanosine or adenosine during viral replication. This leads to the accumulation of malicious errors throughout the viral genome, which ultimately makes the virus non-infectious and unable to replicate. However, prolonged use of this drug can cause liver disease and, moreover, the analysis of SARS-CoV-2 nucleocapsid antibodies does not detect the presence of vaccine-generated neutralizing antibodies to SARS CoV-2 spike protein (S) [1].According to Jia We et al. (2021) seroconversion to viral spike antigens and nucleocapsid antigens usually occurs within 1-3 weeks after infection with SARS-CoV-2, and peak antibody levels reach 4-5 weeks, then every 2 patients were PCR positive (95% CI - 1.5-5.6) [5,6].Monoclonal antibodies such as bamlanivimab-etsevimab-casirivimab-imdevimab and sotrovimab are currently approved for the treatment of patients with SARS-CoV-2, however, the use of such drugs is effective in the initial stage of coronavirus infection, and in addition, serious side effects on the liver are a disadvantage of this kind. treatment [3].Currently, more and more attention is paid to natural sources of biologically active substances in the treatment of coronavirus infection. So, for example, Baicalin, proposed for the treatment of SARS-CoV-2 (Chingju Lin et al, 2021), isolated from the plant Scutellaria baicalensis, has anti-inflammatory, antioxidant and antiviral effects. A decrease in oxidative stress under the influence of baicalin can reduce the pathogenetic effect of the “cytokine storm” and, accordingly, reduce the risk of complications of COVID-19 [8].There is also a growing focus on another natural product, the well-known mushroom G. Lucidum. The composition of this natural product is very wide, including superoxide dismutase, which also reduces the pathogenetic effect of the “cytokine storm” without causing side effects on the liver [10].Also, in recent years, more and more attention has been paid to another natural product of Alkhadaya, which is black cumin oil. I noticed that both G. Lucidum and Alkhadaya contain carboxyl groups in their composition, and they are not a continuation of nitro groups and sulfhydryl groups. These carboxyl groups originate from the phenolic rings of G. Lucidum and the benzene rings of Alkhaday, and the idea of creating a new drug based on G. Lucidum and Alkhaday was proposed, and given its importance not only in the treatment of coronavirus infection, but also in its safe use, this study is considered a hot topic and requires further study.
2. The Aim of the Study
- To conduct a comparative prospective randomized study of the treatment of SARS CoV-2 coronavirus infection with a new drug based on G. Lucidum and Alhadai.
3. Materials and Research Methods
- To achieve this goal, the results of treatment of 200 mature rats of both sexes were analyzed. 160 rats were infected with a coronavirus infection. The maintenance of animals, surgical interventions and withdrawal from the experiment were carried out on the basis of ethical principles declared by the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Purposes. The animals were kept in a vivarium with free access to food and water and a natural change of day and night. The experiments were carried out under conditions of spontaneous respiration and an ambient temperature of 24-25°C. Virus isolation was performed on a vitro cell culture from a virus-containing sample of clinical material (nasopharyngeal swab). The efficiency of replication of the SARS-Cov-2 virus in cell culture was assessed by the dynamics of the appearance of cytopathic action and the presence of viral RNA in the analysis of the culture fluid by polymerase chain reaction (PCR).Rats were intranasally challenged with SARS-Cov-2 strain at 50% tissue culture median infectious dose (TCID 50) per 50 µl inoculum (live culture biologic) after intraperitoneal anesthesia using 2.5% sodium thiopental solution.All animals were divided into 5 groups.Group 1 - absolutely healthy rats (n=40).Group II - rats infected with coronavirus infection treated with remdesavir (n=40).Group III - rats infected with coronavirus infection treated with molnupiravir (n=40).Group IV - rats infected with coronavirus infection treated with baicalin (n=40).Group V - rats infected with coronavirus infection treated with a new drug based on G. Lucidum and Alkhadai (n=40).Remdesavir is a traditional drug used at a dosage of 20 mg/kg of body weight.Molnupiravir is known to be an experimental oral form of a potent ribonucleoside analogue that inhibits the replication of SARS-CoV-2, the causative agent of COVID-19. In this study, Molnupiravir was used at a dose of 25 mg/kg body weight.Baikalin was obtained from the Baikal skullcap with a particle size of 0.1-0.5 mm. The extraction method used is simple maceration for a specified period of time, with a ratio of raw materials: extractant 1:10 m / o and a temperature of 24±1°C containing water begins to actively hydrolyze baicalin to its aglycone (baicalein) and glucuronic acid. Baicalin has a stimulating effect on the glutathione link of NADPH - GSH-dependent AOS: it increases the content of reduced glutathione in the liver in healthy animals and the activity of glutathione synthetase and glucose-6-phosphodehydrogenase in the liver and kidneys under conditions of oxidative stress under the action of various toxicants. Baicalin was administered to rats intraperitoneally at a dose of 15 mg/kg of body weight.A new drug based on G. Lucidum and Alkhadai was administered intraperitoneally at a dose of 100 mg/kg of body weight.The amplification reaction and analysis of PCR products were carried out in the mode real time cycler "Rotor-Gene 6000" ("Corbett Research", Australia). The reaction mixtures included oligonucleotide forward and reverse primers complementary to a specific fragment, fluorescent probes labeled with the FAM fluorophore (carboxyfluorescein) and fluorescence quencher (RTQ1), deoxyribonucleoside triphosphates (dNTPs), MgCl2, buffer, Taq polymerase enzyme, and deionized sterile water. For the negative control, the same volume of distilled water was added to the test tube instead of the sample.Positive samples were determined by the presence of a phase of the logarithmic growth of the fluorescence curve. Registration of results in real time (the value of the threshold cycle, Ct) was performed in tabular and graphical form using computer programs.Statistical processing was carried out taking into account parametric and nonparametric research methods.
4. Research Results
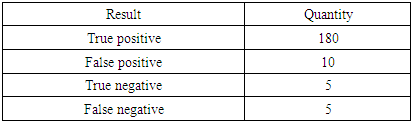
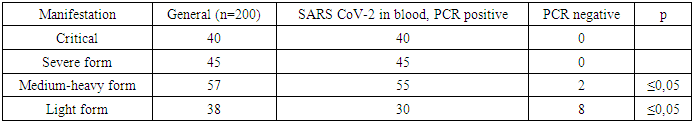
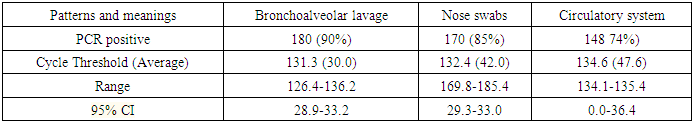
- After infection of rats with coronavirus infection COVID-19, a routine blood test of 200 rats for the presence of the SARS CoV-2 virus was performed by taking scrapings from the oropharynx and bronchoalveolar lavage. A 500 copy/ml sample was used to detect coronavirus infection. Table 1 before treatment shows the results of a PCR test after contracting a coronavirus infection.
|
|
|
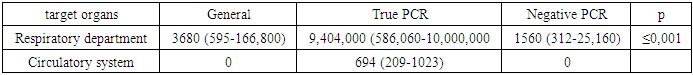
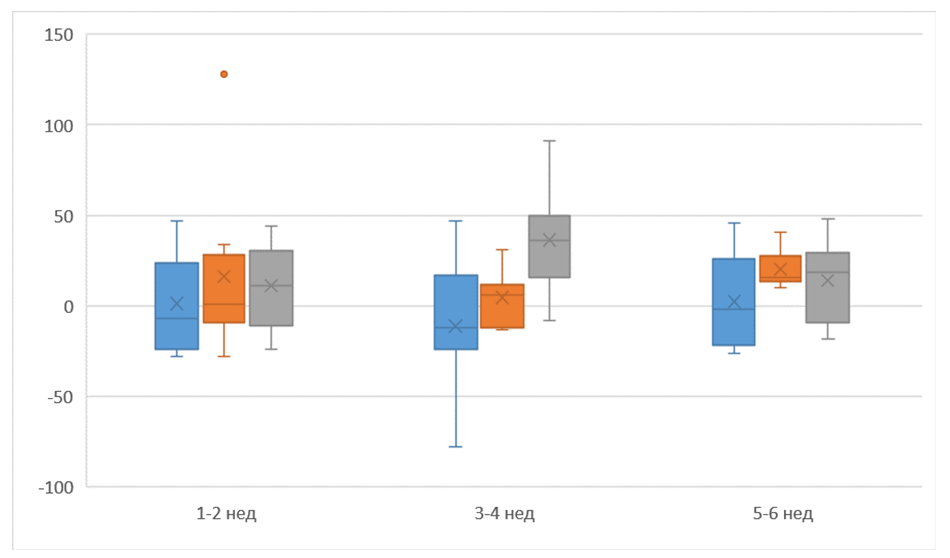 | Figure 1. Changes in the respiratory and circulatory systems under the influence of coronavirus infection and their pathogenetic relationship (Kruskes-Wallis criterion) |
|
5. Discussion and Conclusions
- This virus is known to infect humanity by airborne droplets and the minimum amount is sufficient for infection. Getting through the respiratory system into the bloodstream, this virus encounters a huge number of stages of immune defense on its way, but this virus has learned to evade them. So, this virus entering the body is not detected by our system due to the heterogeneous variability of the virus, a large number of anti-immune mechanisms and an extremely low amount of antibody titer (IG G / Ig M) produced by our body. And all because the HLA antigens of our body cannot recognize this antigen due to the fact that Toll-kike receptors of macrophages do not contain the proper amount of N-galactosamine necessary for recognition of this virus. In addition, 7-transmembrane G-protein coupled receptors that activate an alternative pathway of macrophage activation through the complement system also cannot recognize this virus due to the genetic rearrangement of this virus [1]. In addition, Toll-like receptors present on the surface of almost all immune cells do not recognize this virus as antigenic because of the receptors contained in the glycopeptide envelope of this virus, which, as soon as they recognize the antigens of the host organism, immediately begin to express polypeptides equivalent to amino acid sequences. our body [2]. In addition, the viral capsid membrane has a very powerful defense mechanism. Thus, the receptors in this shell instantly react to antibodies secreted by B cells and neutralize these antibodies, and the antigen-antibody complex itself is not formed, which is not further presented to macrophages and is not presented to antibody-producing cells. Also, this virus is able to actively multiply due to uncontrolled growth rates. So, this organism lacks the Hayflick limit, which, as you know, helps many malignant tumors to multiply uncontrollably. Toll-lke receptors, as already mentioned, are not able to determine this virus as pathogenic with high virulence, and also these receptors, according to the feedback principle through the ligand-activated ubictivin-proteasome pathway, are not able to activate the WNT/β-catenin signaling pathway, which is the main signaling pathway for cell repair. 7-transmembrane G-protein coupled receptors are also not able to properly activate and thus neither TH1 nor TH2 cells are activated and of course there is no leukocyte rolling bond as Syalil-Lewis X-glycoprotein is not activated via cofactors and ligands [3]. Also, even if a small number of leukocytes are activated and migrated through the blood vessels, this amount is clearly not enough to kill this virus. Cytokines, such as the TNF and Ig superfamily, interleukins (IL-1, IL-6β, IL-10) known as anti-inflammatory cytokines also do not have proper killing properties due to insufficient activation of these substances, which, by the feedback principle, also do not activate further presentation of an antigen on antigen-presenting cells. MHC cells of type 1 and type II are also unable to activate independently, since there is no signal received by these cells through ligand-mediated interactions between dendritic cells and macrophages [4-7]. Further activation of macrophages through alternative and lectin pathways does not stabilize the process, and reactive oxygen species (ROS) and free radicals such as chlorine and nitrogen do not form strong antidetoxifying substances such as peroxynitrite and hypochloride. Thus, currently used substances such as chloroquine just act to activate free forms of radicals. Thus, chlorine, which is part of chloroquine due to stimulation of Na + - K + ATPase, cannot be used in salt-sensitive patients due to an increase in the release of Na + from the cell along with chlorine and water. The fluid accumulates in the interstitial space and can lead to serious complications, such as anasarca, etc. Also, Cl-, which is part of many drugs used to treat COVID-19 coronavirus, has an uncontrolled bactericidal effect and can be harmful in severe conditions. Also, for an effective Na + - K + pump system, it uses a large amount of ATP to enter K + into the cell. Ganoderma Lucidum can activate the production of ATP in mitochondria through energy-dependent channels through which a part of adenosine is transferred to the organelles themselves using carrier proteins. Ganoderma Lucidum also activates carriers of Zn dependent channels, through which zinc is transferred to mitochondria, through which ATP is formed [8-10]. Further, Ganoderma Lucidum activates the enzymes involved in the synthesis of vitamin A (retinol) through its influence on retinoic acid receptor (RAR) ligands, causing their differentiation, i.e. provitamin is synthesized into vitamin. Thus, RAR receptors enhanced under the action of Ganoderma Lucidum lead to virus inactivation. Since vitamin A is a fat-soluble vitamin and β-carotene is required for its formation, Alkhadaya or black cumin oil is a great source of cholesterol and polyunsaturated fatty acids, which are converted in the body first into LDL (low density lipoproteins), which are then converted into HDL (lipoproteins). high density). Ganoderma Lucidum also affects the amount and composition of matrix metalloproteinases, in particular (MMP-3 and MMP-9), which are formed in large quantities in this type of virus [1,3]. Thus, Ganoderma Lucidum activates the tissue inhibitor of matrix metalloproteinases (TIMMP), by influencing transforming growth factor β (TGF-β) through the activation of mast cells and macrophages. As is known, matrix metalloproteinases have a Zn-bound domain on their surface and destructive components of the ECM (intracellular matrix). Since when exposed to this virus, procollagenases are not formed, and therefore it is not activated by free radicals for further transformation into collagen. Ganoderma Lucidum also affects the membrane-bound ADAM metalloproteinases, which control the undesirable effects of these proteases through tissue inhibitors of matrix metalloproteinases. Since ADAM deficiency leads directly to lung hypoplasia, then of course the influence of Ganoderma Lucidum and the strengthening of its function leads to non-union of the alveoli and the formation of connective tissue, which the virus controls so much. Also, as a result of gene rearrangement and increased expression of this virus, tyrosine kinase-type receptors are activated. It also turns out that the host organism stimulates the production of more of this virus due to the inability of the parafollicular C-cells of the thyroid gland to keep these receptors in balance. There is a kind of constant demirization and activation of its receptor in an initially incorrect chemical formula, localized in the cytoplasmic catalytic domain, and thus the substrate specificity of tyrosine kinase is violated.
6. Conclusions
- Thus, the use of a new drug based on G. Lucidum and Alkhadai is effective against coronavirus infection caused by COVID-19 and has no side effects, as proven by the study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML