Sarkisova Lyalya Valerievna
Bukhara State Medical Institute named after Abu Ali ibn Sina, Bukhara, Uzbekistan
Correspondence to: Sarkisova Lyalya Valerievna, Bukhara State Medical Institute named after Abu Ali ibn Sina, Bukhara, Uzbekistan.
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The article presents the results of a genetic study of 223 women at 22-36 weeks of gestation, including 65 women with a risk of miscarriage, 52 with premature birth, and a control group of 106 pregnant women. The polymorphism of the IL-1β (T31C), TNF-α (G308A) and IL-10 (G1082A) genes was studied using the Rotor-Gene Q device. The material was DNA isolated from the peripheral venous blood of pregnant women. The studies have shown that the polymorphism of the IL-1β (T31C) and IL-10 (G1082A) genes are markers for predicting premature birth.
Keywords:
Premature birth, Risk factors, Genetic markers, Allele, Genotype, Mutant allele, Homozygote, Heterozygote, Polymorphism of the IL-1β (T31C), TNF-α (G308A) and IL-10 (G1082A) genes
Cite this paper: Sarkisova Lyalya Valerievna, Diagnostic Significance of Genetic Predictors in Predicting Premature Birth, American Journal of Medicine and Medical Sciences, Vol. 14 No. 9, 2024, pp. 2372-2375. doi: 10.5923/j.ajmms.20241409.53.
1. Introduction
All over the world, premature birth (PB) is one of the most important aspects of the problem of protecting the health of mother and child, being a severe pathology of pregnancy, the prevalence of which increases every year, despite the successful development of science [3,5,7]. According to the World Health Organization (WHO), in economically developed countries, premature birth is the outcome of 5-12% of all pregnancies. In Russia, the frequency of PB was 5-10% and has not shown a tendency to decrease over the past 10 years [6,8]. According to world literature, at the beginning of the 21st century, this figure in the USA was 10.1%, in England - 7.8%, in France - 7.2%. [1,3,11]. In recent years, the incidence of premature births in Uzbekistan has remained within 9-15%, in addition, about 70% of cases of early neonatal mortality are associated with prematurity [9,10,12]. In this regard, in recent years, scientists have increasingly focused on the search for genetic predictors of premature births in order to predict and timely select obstetric tactics, in order to reduce perinatal mortality and material costs for therapy and rehabilitation of newborns [2,4]. A number of reforms and scientific research have been carried out in our republic aimed at organizing a health care system based on advanced world standards, providing high-quality medical services to the population, as well as early diagnosis and prediction of obstetric and gynecological diseases that occur among the population. In this regard, within the framework of the implementation of measures to reform the healthcare system in the republic, special attention is paid to "... improving the system of maternal and child health care based on the development of medical genetics, emergency and specialized medical care for women and children, the introduction of modern screening programs, the creation of multidisciplinary medical complexes and information systems "Mother and Child ..." in the regions. In order to improve the effectiveness of medical and social care for pregnant women with threatened premature birth and with premature birth, when implementing the set tasks, it is important to reduce the level of disability, improve the quality of life of women, improve the methods of using modern technologies in the provision of high-quality medical services.
2. Materials and Methods
We studied the course of pregnancy and childbirth of 223 women at a gestation period of 22-36 weeks, including 65 women with a threat of termination of pregnancy, 52 with premature birth. The control group included 106 pregnant women.Genetic studies were carried out at the Republican Research Medical Center of Hematology in the Department of Molecular Cellular Medical Technology. The studies were carried out on the Rotor-Gene Q device. The material was DNA isolated from the peripheral venous blood of pregnant women. All women in the observed main group underwent a genetic study of the polymorphism of the IL-1b (T31C), TNF-α (G308A) and IL10 (G1082A) genes.
3. Results and Discussion
We studied the frequency distribution of alleles and genotypes of the IL-1b (T31C) TNF-α (G308A) and IL-10 (G1082A) gene polymorphism in a group of pregnant women with threatened preterm labor, with preterm labor and normal pregnancy included in our study. In our study, we found that in patients of the main group, homozygous TT or wild-type genotype of the IL-1b (T31C) gene was in 31.6%, heterozygous TC genotype in 43.6%, homozygous CC CC or mutant form genotype in 24.8% of pregnant women.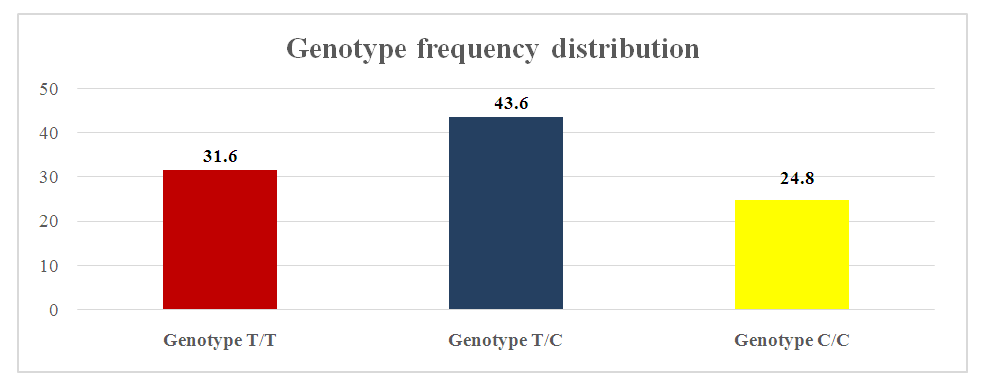 | Figure 1. Distribution of genotypes of the T31C polymorphism in the IL-1b gene in pregnant women of the main group |
The frequency of IL-1β gene alleles was as follows: in the main group, the proportion of T allele and mutant C allele was 53.4% and 46.6%, while in the control group these figures were 58% and 42%, respectively, according to the established ratio. For T allele, respectively (χ2=1.0; p>0.4; PP=0.9; 95% CI: 0.65–1.3; OR=0.8; 95% CI: 0.57–1.21). For mutant C allele, respectively (χ2=1.0; p>0.4; PP=1.1; 95% CI: 0.74-1.6; OR=1.2; 95% CI: 0.83-1.75). Regarding the OR, the mutant allele C increases the risk of preterm birth in pregnant women included in the study (OR = 0.53; 95% CI: 0.3–2.15). Our results for both allelic genes were reliable (χ2 = 4.5; p = 0.05). The T/T, T/C, C/C T31C genotypes in the IL–1β gene in pregnant women in the main group were 31.6%, 43.6%, and 24.8%, compared with the control group, these figures were 35.8%, 44.3%, and 19.8%. In the main group, it was found that heterozygous T/C and mutant C/C genotypes prevailed, with T/C (χ2=0.0; P>0.95; PP=1.0; 95% CI: 0.6–1.61; OR=1.0; 95% CI: 0.57–1.65), and the mutant C/C genotype (χ2=0.8; P>0.4; RR=1.3; 95% CI: 0.73-2.16; OR=1.3; 95% CI: 0.71–2.52) being the ratio. The presented data show that in our study, pregnant women with the mutant variant of the C/C genotype had a significantly increased risk of premature birth (OR=1.3; 95% CI: 0.71–2.52). Thus, since the AUC on average is 0.76, mutant allele C, C/C heterozygote of the IL-1β gene has a relatively high prognostic efficiency as a marker for predicting preterm birth. In our study, the distribution of genotype frequencies in the IL1b gene (T31C) in the main and control groups obeyed the Hardy-Weinberg law. We were able to find a statistically significant association between the polymorphism and an increased risk of preterm birth, for the wild-type allele and non-wild-type allele, as well as for other genotypes. With the exception of the second group, when the wild-type allele (T) and non-wild-type allele (C) showed a reliable and significant result as a proactive and initiative role. Some studies suggested that the T allele of the polymorphism at position 31 (IL1β-31T) is located at the start of transcription and is also associated with a decrease in IL-1β production. This means that IL1b is expressed more by the C allele compared to the T allele. In particular, IL-1b has been shown to induce the expression of prostaglandin H synthase 2 (PGHS-2, also called COX-2), an enzyme that catalyzes the synthesis of prostaglandins (eg, PGE2, PGF2a) from arachidonic acid, in humans during pregnancy. These prostaglandins are known to cause cervical ripening and myometrial contractions. Therefore, the T allele of the IL1b gene (T31C) may overexpress the cytokine IL-1b, which leads to preterm labor due to myometrial contractions more often and earlier than normal. This assumption may explain why the C allele was more common in the study group compared to the control group in our study.The TNF-a gene in the main group of pregnant women with the wild homozygous genotype G/G was 88%, and with the heterozygous genotype G/A–12%, respectively, in the control group–88.7% and 11.3% of pregnant women were equal. No gomozygous mutant variant A/A was detected in any of the groups.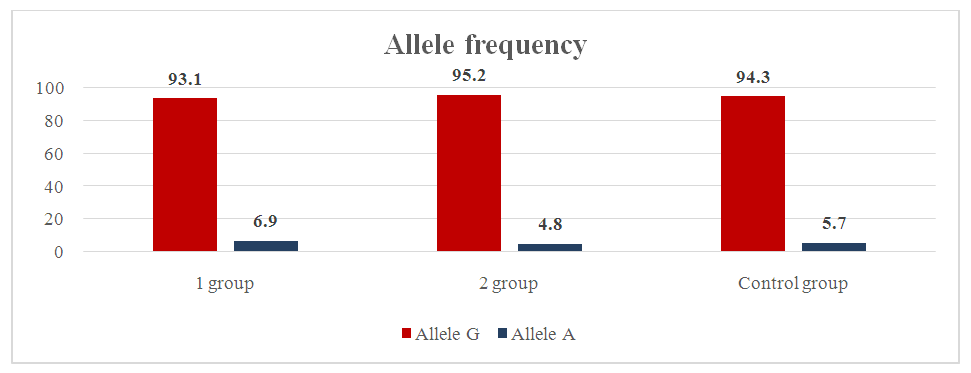 | Figure 2. Frequency of distribution of alleles of gene polymorphism genotypes TNF-a in in the main and control groups |
The proportion of mutant allele A was 12% in the main group and 14% in the control group. For allele G, respectively (χ2=0.0; p=0.9; RR=1.0; 95% CI: 0.49–2.05; OR=0.9; 95% CI: 0.43–2.09). For mutant allele A, respectively (χ2=0.0; p=0.9; RR=1.0; 95% CI: 0.43–2.32; OR=1.1; 95% CI: 0.48–2.35).In the prognostic model of alleles of the TNF-α gene, it was found that the sensitivity of mutant alleles in the main group showed a good result of 94%. The frequency distribution of alleles and genotypes of the IL-10 gene polymorphism (G1082A) was studied in a group of pregnant women with a risk of premature birth, complications of premature birth, and normal pregnancy. The homozygous genotype G/G was 17.9%, the heterozygous genotype G/A was 39.3%, and the homozygous mutant form A/A was 42.7% in the main group of pregnant women with a risk of premature birth and complicated premature birth. In pregnant women in the control group, these genotypes were 3.8%, 31.1%, and 65.1%, respectively. In our study, the frequency of the wild-type G allele and the mutant A allele was 37.6% and 62.4% in the main group and 19.3% and 80.7% in the control group, respectively. For the G allele, respectively (χ2=18.1; p=0.01; PP=1.9; 95% CI: 1.4–2.7; OR=2.5; 95% CI: 1.64–3.85). For the mutant A allele, respectively (χ2=18.1; p=0.01; RR=0.5; 95% CI: 0.3–0.88; OR=0.4; 95% CI: 0.26–0.61). As for the OR, the mutant A allele increases the risk of preterm birth in pregnant women included in the study (OR=0.4; 95% CI: 0.26–0.61). The statistical analysis we obtained for both allelic genes was reliable (χ2=18.1; p=0.01). The genotypes G/G, G/A, A/A of the IL-10 gene (G1082A) in pregnant women of the main group were 17.9%, 39.3% and 42.7% compared to the control group, 3.8%, 31.1%, 65.1%, respectively. In the control group, the frequency of detection of the G/G wild genotype was slightly higher and, accordingly, (χ2=11.2; p=0.01; RR=4.8; 95% CI: 3.07-7.37; OR=5.6; 95% CI: 2.04-15.25). The same ratio of the heterozygous genotype G/A was noted in the main and control groups and, accordingly (χ2=1.6; p=0.3; RR=1.3; 95% CI: 0.77-2.06; OR=1.4; 95% CI: 0.82-2.49), it was established that the mutant genotype A/A was superior to the control group and, accordingly (χ2=11.2; p=0.01; RR=0.7; 95% CI: 0.4-1.08; OR=0.4; 95% CI: 0.23-0.68) – ratio.When dividing the main group of pregnant women into two subgroups, we included pregnant women with a risk of premature birth in the first subgroup, that is, the G allele of the IL-10 gene was 36.9%, the A allele was 63.1%. 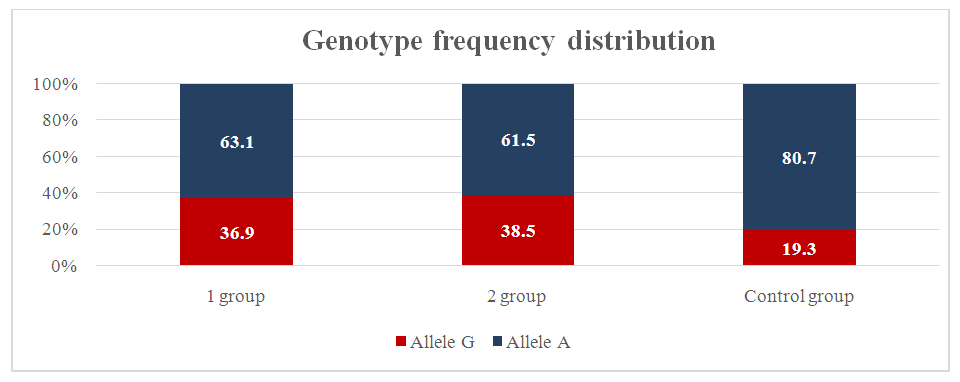 | Figure 3. Frequency of distribution of alleles of genotypes of the IL-10 gene G 1082A polymorphism in the main and control groups |
In the control group, these indicators were 19.3% and 80.7%, respectively. Compared with group I, the proportion of G alleles in the control group was slightly higher (χ2=12.9; p=0.01; RR=1.9; 95% CI: 1.14-3.19; OR=2.4; 95% CI: 1.5-3.97). And the indicator of the A allele was slightly higher in group I compared with the control group, respectively (χ2=12.9; p=0.01; RR=0.5; 95% CI: 0.33-0.84; OR = 0.4; 95% CI: 0.25-0.67). From the above indicators, it was clear that the mutant allele A, in turn, significantly increases the likelihood of premature birth. The wild G allele plays a protective role. The results of our study were statistically significant (χ2=12.9; P=0.01). The G1082A polymorphism of the IL-10 gene in groups I and II, an indicator of the prevalence of the wild-type G allele, was found with the same frequency in pregnant women of group I (pregnant women at risk of premature birth) and group II (pregnant women with complications of premature birth) 36.9% and 38.5%, respectively. The mutant allele A was found equally often in both groups, 63.1% and 61.5%, respectively. In group II, the prevalence of the homozygous wild genotype G/G was revealed compared to group I (20% and 15.4%, respectively; (χ2=6.7; p=0.01; RR=4.1; 95% CI: 1.62-10.24; OR=4.6; 95% CI: 1.45-14.81). In group I, compared to group II, the frequency of detection of the heterozygous genotype G/A was higher, the analog indicators were higher by 33.8% and 46.2%, respectively (χ2=3.4; p=0.1; RR=1.5; 95% CI: 0.63-3.5; OR=1.9; 95% CI: 0.96-3.74). Compared with group I, group II showed a higher frequency of mutant A/A homozygous genotype, the ratio was 46.2% to 38.5% and, accordingly, (χ2=10.1; p=0.01; RR=0.6; 95% CI: 0.24-1.46; OR = 0.3; 95% CI: 0.17-0.66).
4. Conclusions
In our study, although the distribution of genotype frequencies in the TNFa gene (G308A) in the main and control groups obeyed the Hardy-Weinberg law, as in the case of the IL1b gene (T31C), in the TNFa gene we did not reveal a statistically significant association between the mutant form of the TNFa gene (G308A) and premature birth in the Uzbek population (χ2<3.84; P>0.05). In particular, we could not find significant differences between the main and control groups (as well as the two subgroups) in the distribution of the mutant allele and heterozygous genotypes. In addition, in our studies, the homozygous mutant genotype was not detected in either the control or the main group. Thus, the prognosis of preterm birth development by the mutant allele A of the IL-10 and IL-1b gene has a high prognostic efficiency. IL10 can play a protective role against preterm birth by suppressing the immune response and maintaining embryonic tolerance. But in the alternative or non-wild type, the IL10 gene can limit the expression of this cytokine, which leads to an imbalance between the proinflammatory and anti-inflammatory response. As a result, when the immune response is insufficiently suppressed by IL-10, it can cause an increase in the inflammatory process in the uterus. This proinflammatory environment promotes uterine contraction, expulsion of the child and rejection of the placenta, which leads to preterm birth. It can be concluded that the mutant form of the A/A genotype is important in the development of preterm birth in the main group and can be a predictor of preterm birth in women of the Uzbek nation.
References
| [1] | Volkov V. G., Chursina O. V., Modern Possibilities of Predicting Preterm Birth. Bulletin of New Medical Technologies. Electronic publication - 2020 - No. 1 - P. 30-35. |
| [2] | German A. I. Risk factors for premature birth "Actual problems of modern medicine and pharmacy - 2020" - P. 34-37. |
| [3] | Gorina K. A. Optimization of obstetric tactics in patients at high risk of premature birth based on a comprehensive assessment of clinical and molecular biological factors / Moska 2021 - P. 3-13. |
| [4] | Musalaeva I. O., Tarasenko E. V., Kostin I. N., Azova M. M., Olenev A. S. Preterm birth: new possibilities for forecasting Obstetrics and Gynecology Vol. 8 No. 3- 2020. P. 10-14. |
| [5] | Radzinsky V. E., Orazmuradov A. A., Savenkova I. V., Damirova K. F., Haddad H. Preterm birth - an unsolved problem of the 21st century. Kuban Scientific Medical Bulletin. 2020; 27(4). - P. 27–37. |
| [6] | Fedotovskaya O. I. Optimization of obstetric tactics in preterm birth - The role of clinical and molecular genetic factors / Moscow 2014 Abstract. |
| [7] | Xian-Ling Cao., Xuan-You Zhou., Song-Chang Chen., Chen -Ming Xu. Association of Il-4 and Il-10 Polymorphisms With Preterm Birth Susceptibility: A Systematic Review and Meta-Analysis. 2022. Journal Frontiers in Immunology. 2022. - Vol. 13. Article 917383. P. 1-1. |
| [8] | Qin Zhu., Jian Sun., Ying Chen. Preterm birth and single nucleotide polymorphisms in cytokine genes // Journal Translational Pediatrics. 2014; 3(2) P. 120–134. |
| [9] | Sarkisova L. V., Inoyatov A. Sh. The role of genetic predictors in predicting preterm birth. Tibbiyotda Yangi Kun 2022. - No. 6, - P. 219-224 10. |
| [10] | Sarkisova Lyalya Valerievna Modern Approach of Assessment of the Risk of Preterm Birth. American Journal of Medicine and Medical Sciences 2021 (Scopus), 11 (9): - P. 630 -634. |
| [11] | Lyalya Sarkisova Valerievna. Assessment of the risk of preterm birth by assessing the polymorphism of tumor necrosis factor alpha and interleukin 1-beta. Journal of Natural Remedies (Scopus). Vol. 22, No. 1(2), (2021) P. 95-101. |
| [12] | Lyalya Sarkisova, Nilufar Karimova, Nigina Hikmatova, Botur Shodiyev, Madina Kadyrbayeva and Gulrukh Suleymanova. BIO Web of Conferences 121/04011(2024) Immunological predictors bin predicting premature birth. (Scopus). |





 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML