-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(9): 2348-2350
doi:10.5923/j.ajmms.20241409.47
Received: Aug. 29, 2024; Accepted: Sep. 16, 2024; Published: Sep. 28, 2024

The Practical Significance of the VEGF Factor in the Acute Period of Ischemic Stroke
Tashkenov E. M.
Andijan State Medical Institute, Andijan, Uzbekistan
Correspondence to: Tashkenov E. M., Andijan State Medical Institute, Andijan, Uzbekistan.
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Stroke disease has a profound effect on the economy and health of the whole world and our country. In recent times, the diagnosis of stroke is focused on the dilution of laboratory methods, that is, it is introduced as a new approach in the study of the pathogenesis of the disease.
Keywords: Ishemik insult, VEGF omillari, Ion tomirlar endoteliysi, Metabolizm
Cite this paper: Tashkenov E. M., The Practical Significance of the VEGF Factor in the Acute Period of Ischemic Stroke, American Journal of Medicine and Medical Sciences, Vol. 14 No. 9, 2024, pp. 2348-2350. doi: 10.5923/j.ajmms.20241409.47.
1. Introduction
- Concepts about metabolism pathways expand and biomarkers involved in pathological processes lead to an increase in the amount of therapeutic targets and the opening of new doors in the future in medicine [1,2,3]. Biomarkers found in cerebral stroke include S100 protein, neuronal specific enolase (NSE), NMDA receptor protein or antibodies, basic myelin protein (MBP), s reactive IQs (SRP), interleukin 6 (IL6), Matrix metalloprotease (MMR2 and MMR9), and X. The production of an endothelial growth factor in the vascular endothelium increases when the brain infartis lacks oxygen to the tissue. Vascular endothelial growth factor or VEGF-A is part of the most studied group of proangiogenic factoryl. The endothelial growth factor is a potent stimulant of angiogenesis among the waxes of the Kup-numbered VEGF family. The VEGF gene is located on the short shoulder of chromosome 6, with receptors divided into 4 classes: VEGFR1, VEGFR2, VEGFR3, VEGFR4. These receptors are located primarily in endothelial cells, with small amounts in megacariocytes, monocytes, neurons, spindle hematopoietin, and tumor cells. VEGFR1 has been little studied, preventing it from binding to VEGF and VEGFR1, blocking the intracellular reaction cascade, lowering vascular permeability, reducing inflammation and angiogenesis [7,8,9]. VEGFR2 is the primary receptor, carrying out the transfer of the activated signal. This receptor binds to VEGF and stimulates intracellular proteinkinase. VEGFR2 activation increases endothelial cell differentiation, migration and proliferation, increases vascular permeability, promotes changes in inflammation, binds to neoangiogenesis and atherosclerosis, mobilizes endothelial cells. VEGFR3 is involved in lymphogenesis. In a mature organism, VEGF is located in all vascular tissues and is involved in the support of vascular hemostasis. This feature is the VEGF vasculoprotective effect: ensuring the formation and survival of endothelial cells, production of NO and prostacyclin, vasodilation, antithrombosis and proliferation of smooth muscle cells, activation of chemotaxis, support changes in the inflammatory process [16,17,18]. Under mechanical forces, as a result of the transformation, inflammation, hypoxia of the structure of the cell, the production of the VEGF factor begins under the influence of vasoactive hormones angiotensin II and vasopressin. Under tissue hypoxia conditions, VEGF does not wait for expression, but imposes an increase in capillary density and a low drop in arterial pressure. The VEGF factor ensures not only the formation of normal blood vessels, but also the formation and survival, and is also involved in the regulation of blood pressure. Prolonged vact causes decreased survival of endothelial cells, decreased terminal capillary and arterioles in tissues, increased blood pressure as a result of reduced VEGF levels in the blood or VEGFR receptor blocks. VEGF is a potent angiogenic glycoprotein, producing from a vascular endothelial cell, macrophages, neurons, and neuroglia in response to cerebral hypoxic damage [1,2,3,9,10].The purpose of the work: to study the role of the VEGF factor in the acute period of ischemic stroke.
2. Materials and Methods
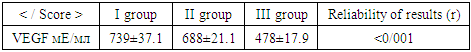
- We selected patients in a private hospital affiliated with Asaka neurology hospital LLC, conducted their clinical neurological examinations, laboratory tests of the Andijan region AIDS karshi wrestling Center in the study of blood VEGF levels. Venous blood serum of selected patients was taken. 3ml of venous blood was taken between 9.00-11.00 am of the day of the collected venous blood, and the Vakutayner containing the Clot Activator was delivered to the examination laboratory for 1.5-2 hours at the same time as turgi adhered to the transportable coids. The laboratory vacuum cleaner was made up of a marked composition, then passed through 3000 speed/min centrafuga for 15 minutes, after which the serum was separated into an Epindorff plate test tube and-200s was frozen and sung in a sacking burn maxad for the IFA inspection method. To perform the IFA examination, we used the" VEGF-IFA-Best " test kit to anise the amount of VEGF (Vascular endothelial growth factor/endothelial growth factor) from acute cranial ischemia and blood serum from healthy group patients. The norm also equates to 691 me/ML under 20-50 years of age in the blood and the examination was carried out using Multiskan™ FC type 357 aparati (Thermo Fisher Scientific). It was also examined with patients ' NIHSS scale, Sollin index and Glasgow scale. A total of 89 patients were selected. We have divided into three groups of all patients. they are 25-76 years old with an average age of 56±1.5. The first group of patients consists of 32 patients who are hastened by the type of acute ischemic atherothrombotic stroke, of which 7 patients are women, 25 patients are men. The second group consisted of 29 patients with acute cerebral ischemia, 15 of them female patients, 14 male patients as well as a third group of 28 healthy individuals with 14 female and 14 male selectively.
3. Results
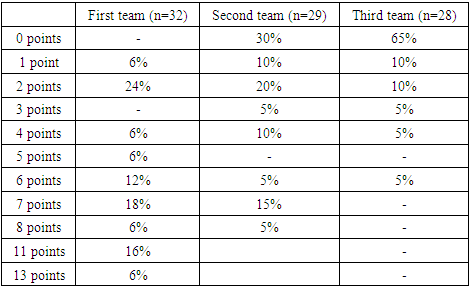
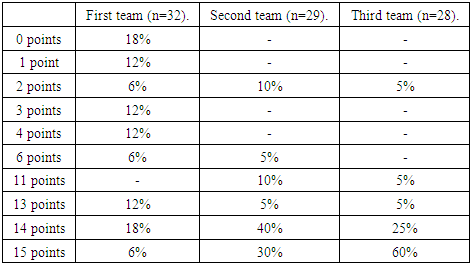
- We used the manna-Uitna method in maksadi to compare the results of the three groups obtained.When the first group (n=32) tested patients on the NIHSS scale, 2 (6%) Scored 1, 7(24%) scored 2, 2(6%) scored 4, 2(6%) scored 5, 4(12%) scored 6, 6(18%) scored 7, 2(6%) Scored 8, 5(16%) scored 11ball, 2(6%) scored 13 (Table 1).
|
|
|
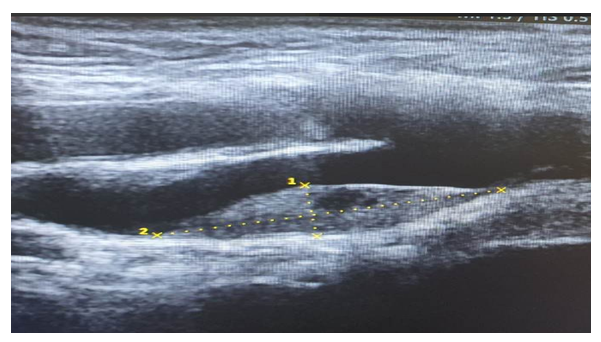 | Figure 1. Left-sided common sleep artery in the intima and medial floors of low-density atherosclerotic plaque occlusion 77% |
4. Conclusions
- In the etiopathogenesis of acute cerebral ischemia, VEGF plays a role in neurospecific oxyl muxime, that is, an increase in the amount of VEGF in the blood during the acute period of patients with acute stroke is due to the chukurity and exacerbation of the disease process. It is also an important factor in assessing and predicting the effectiveness of the treatment of the disease.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML