-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(9): 2328-2332
doi:10.5923/j.ajmms.20241409.43
Received: Aug. 21, 2024; Accepted: Sep. 20, 2024; Published: Sep. 21, 2024

A New Solution to the Problem of Recurrent Urogenital Candidiasis
Munisa Abdushukurovna Mirsaidova, Malika Turakhanovna Alisheva
Republican Specialized Scientific and Practical Medical Centre of Dermatovenereology and Cosmetology, Tashkent, Uzbekistan
Correspondence to: Munisa Abdushukurovna Mirsaidova, Republican Specialized Scientific and Practical Medical Centre of Dermatovenereology and Cosmetology, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: We observed 20 female patients with recurrent urogenital candidiasis who received activated silica solution as a topical medication for rapid disease progression and prevention of recurrences. Purpose: Our study aimed to improve the effectiveness of the treatment of urogenital candidiasis (UGC) and reduce the number of relapses, given the local immune response. Methodology: The problem is solved by applying activated silica solution as a topical medication for vaginal irrigation used additionally in the developed method of treatment, which includes complex therapy (antimycotic drugs, vitamins, anti-inflammatory therapy). Results: The outcomes of the treatment encompassed a notable reduction in discharge, complete alleviation of itching, and the resolution of inflammatory manifestations such as edema and redness within a 7-day. Additionally, patients reported relief from pain and burning sensations. Bacteriological studies revealed a significant decrease in white blood cell count, the disappearance of key cells, and the activation of local immunity. These positive effects contributed to the elimination of relapses, hastening the regeneration processes, and normalizing the state of vaginal microflora. Conclusion: The comprehensive improvement observed underscores the efficacy of the treatment in addressing various symptoms and promoting overall vaginal health.
Keywords: Recurrent urogenital candidiasis, Activated silica solution, Local immune response, Yeast-like fungi
Cite this paper: Munisa Abdushukurovna Mirsaidova, Malika Turakhanovna Alisheva, A New Solution to the Problem of Recurrent Urogenital Candidiasis, American Journal of Medicine and Medical Sciences, Vol. 14 No. 9, 2024, pp. 2328-2332. doi: 10.5923/j.ajmms.20241409.43.
1. Introduction
- Urogenital candidiasis (UGC) is one of the most urgent and complicated problems in the practice of the modern dermatovenerologist. Its high social significance is due to its wide prevalence, especially among women of reproductive age, and its tendency to recurrent attacks, which worsens the quality of patients’ lives [1,2,3]. Urogenital candidiasis is the second most common disease of the female genital system after bacterial vaginosis (BV) [4]. Worldwide, approximately 10 million women consult gynecologists about vaginitis every year [5]. Many studies [5,6,7,9,26] report that three-quarters of these women (75%) have already experienced an episode of urogenital candidiasis, at least 50% of them will have a second episode, and 5 to 10% of all women suffer from recurrent urogenital candidiasis, that is, they have ≥4 episodes of urogenital candidiasis per year.There are various methods of topical treatment for vulvovaginal candidiasis and RVVC, which, in addition to the traditional prescription of systemic drugs, apply: clotrimazole 500 mg suppositories once a week or 200 mg suppositories twice a week; up to 70% cure rate in the presence of C.glabrata, resistant to azole antifungal agents, was observed after the use of boric acid 600 mg gelatin capsules daily for 14 days [19,22]. An alternative is topical application of 17% flucytosine cream for 14 days, which has also proven effective in the presence of C.non-albicans, but this method is limited in use due to the high cost of drugs [7].The disadvantage of this method is that the topical application of antifungal drugs does not eliminate frequent relapses of the disease since it does not affect local immunity. Also, the related vaginal microflora is not normalized, which is a key link, since the disturbed microflora aggravates the course of vulvovaginal candidiasis. This method can only temporarily alleviate the condition of patients for a short time; over time, they have complaints again.Our study aimed to improve the effectiveness of the treatment of urogenital candidiasis (UGC) and reduce the number of relapses, given the local immune response, which responds more intensely to excessive growth of opportunistic pathogenic microflora (OPM) than to fungal infection, which may be due to the ability of fungi to suppress local immune defense or other factors that lead to the formation of an inadequate immune response in UGC.
2. Materials and Methods
- Study Design and Participants:A prospective, observational study was conducted involving 20 female patients aged 18 to 45 years who were diagnosed with recurrent urogenital candidiasis (RUC), defined as experiencing four or more episodes of UGC within the past year. Participants were recruited from the outpatient clinic of the Republican Specialized Scientific and Practical Medical Centre of Dermatovenereology and Cosmetology in Tashkent, Uzbekistan. Inclusion criteria included a confirmed diagnosis of RUC based on clinical symptoms (e.g., vaginal discharge, itching, burning) and positive microbiological findings. Exclusion criteria included pregnancy, immunocompromised states, and recent use of antifungal or antibiotic medications within the last 30 days.A control group consisting of 15 female patients with similar inclusion criteria was also included in the study. These control patients received standard treatment with conventional antifungal solutions, such as Betadine or Citeal, applied via vaginal irrigation. The control group was matched for age and clinical severity to the treatment group to ensure comparability of outcomes.Treatment Protocol:The treatment involved daily vaginal irrigation with 150 mL of an activated silica solution, Fatiderm, for 7 days. Fatiderm is an organic mineral water derived from complexes of siliceous minerals found in Uzbekistan. Its composition includes SiO2 (36 mg/L), Na (319 mg/L), K (13 mg/L), Ca (28 mg/L), Fe (0.3 mg/L), Co (0.0002 mg/L), Ni (0.002 mg/L), and trace amounts of Au, Tb, Sm, Dy, Gd, Er, Ho, and Tm. The solution has a pH of 7.9. The irrigation procedure was conducted using a gynecological speculum, with the solution applied directly to the vaginal cavity, retained for 5 minutes, then washed off and dried with a sterile cotton swab.Fatiderm was used as part of a comprehensive treatment strategy that included systemic antimycotic drugs, vitamins, and anti-inflammatory therapies. The activated silica solution was selected for its properties that enhance the regeneration of skin cells and mucous membranes, exert anti-inflammatory effects, and inhibit the growth of opportunistic microorganisms such as Staphylococcus spp., E. coli, and Candida spp. The microelements in Fatiderm were expected to modulate local immunity, thereby increasing resistance to pathogenic microorganisms and aiding in the restoration of normal vaginal pH and microflora balance.According to Costernon J. W. (1995), most of the above infections occur with biofilm formation. Gintsburg A.L., Ilyina T.S. et al. (2003) revealed the role of trace elements, especially iron, copper, potassium, magnesium in metabolic processes that affect biofilm formation. The microelements are involved in the activation of oxidative processes, enzyme systems, and energy production occurring in mitochondria [1,22].Due to biofilms, microorganisms increase their resistance by 50 to 500 times to the action of disinfectants, antibacterial agents, bacteriophages, antibodies, and phagocytes. This phenomenon produced by microorganisms is the main etiopathogenetic factor in the chronicity and frequent recurrence of the disease, which in turn causes antibacterial resistance [25]. A study conducted by Helen Knight at the Massachusetts Institute of Technology found that chelating proteins in the neutrophils of the human immune system play an important role in the fight against infections. One of the main representatives of the protein fraction is the calprotectin protein, which binds zinc, manganese and iron ions, thereby suppressing the spread of both bacteria and infectious fungi. Experimental studies have established that protein depletes iron from the medium, and excess calcium in the medium leads to a far more significant depletion of iron, which stops bacterial growth. Using radioactive isotopes, it was possible to demonstrate that protein prevents microbes from absorbing the desired microelement [1,22].
3. Results
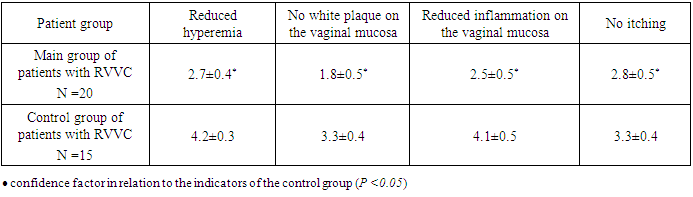
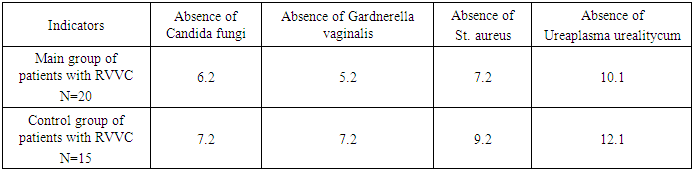
- The study evaluated the effectiveness of activated silica solution (Fatiderm) in 20 female patients with recurrent urogenital candidiasis (RUC). At baseline, all participants presented with elevated leukocytosis and the presence of fungi in vaginal smears, with 11 patients (55%) exhibiting key cells indicative of bacterial vaginosis. Candida albicans was identified in 12 patients (60%), while Candida tropicalis was detected in 8 patients (40%). Common clinical complaints included curd-like vaginal discharge, itching, and burning sensations, with patients experiencing 3-4 relapses per year.Following a 7-day treatment regimen involving daily vaginal irrigation with 150 mL of Fatiderm, significant clinical and microbiological improvements were observed. By the third day of treatment, there was a marked reduction in vaginal discharge and itching, along with the resolution of inflammatory signs such as edema and redness. By the seventh day, bacteriological examination revealed no fungi or key cells in smears, and the leukocyte counts were significantly reduced. All patients reported complete alleviation of itching, pain, and burning sensations by the end of the treatment period. No relapses were observed during a 1-year follow-up, indicating a sustained therapeutic effect.In contrast, the control group of 15 patients treated with conventional antifungal solutions (Betadine or Citeal) showed improvement in symptoms by the 5th to 6th day of treatment. However, fungi were still detected in smears of two patients post-treatment, with Candida albicans present in cultures. All patients in the control group experienced recurrences within the follow-up period, highlighting the potential superiority of Fatiderm in preventing relapses.
|
|
4. Discussion
- The findings from this study demonstrate that activated silica solution (Fatiderm) is highly effective in treating recurrent urogenital candidiasis, achieving rapid symptom relief and microbiological clearance within seven days of treatment. The notable reduction in discharge, itching, and inflammatory signs observed as early as the third day suggests that Fatiderm may offer a faster and more comprehensive therapeutic response compared to conventional antifungal treatments. The complete absence of fungi and key cells in post-treatment smears, along with significant reductions in leukocyte counts, indicate that Fatiderm effectively addresses both the fungal infection and associated bacterial vaginosis, which are often co-factors in the recurrence of UGC.The sustained absence of relapses during the 1-year follow-up period further underscores the efficacy of Fatiderm, potentially linked to its multifaceted mechanism of action, which includes the regeneration of mucosal surfaces, anti-inflammatory properties, and enhancement of local immune response. This is in contrast to conventional treatments, which primarily target the fungal pathogen without significantly impacting local immunity or vaginal microflora. The inclusion of trace elements such as SiO2, Na, and Ca in Fatiderm may play a crucial role in modulating the local immune environment and inhibiting the growth of opportunistic microorganisms, thereby reducing the risk of recurrence.The study’s findings are consistent with the hypothesis that effective management of RUC requires not only antifungal activity but also the restoration of vaginal ecosystem balance and enhancement of host defenses. These results suggest that Fatiderm could serve as a valuable addition to the therapeutic arsenal for RUC, particularly in cases where traditional antifungal therapies have failed to prevent relapses. However, limitations of this study include the small sample size and the lack of a randomized controlled trial design. Future studies should aim to validate these findings in larger, randomized populations and explore the long-term effects of Fatiderm on vaginal health and immune function.
5. Conclusions
- The development of recurrent vulvovaginal candidiasis (RVVC) is a multifactorial process influenced by both the pathogenic properties of yeast fungi and disruptions in the vaginal microflora. These factors contribute to the inflammatory response and the recurrence of urogenital candidiasis (UGC), leading to significant discomfort for patients. Addressing this pathology requires a deeper understanding of the interactions between disrupted microflora and local immune defenses within the vaginal environment. Effective treatment of UGC, especially when associated with microflora disturbances, must therefore adopt a comprehensive approach that targets not only the fungal pathogens but also addresses bacterial opportunistic microflora. Such a strategy is crucial for achieving optimal therapeutic outcomes and preventing recurrent episodes of the disease.Current evidence underscores the necessity of a thorough examination of patients with RVVC, extending beyond mere detection of fungal pathogens to include a detailed assessment of the bacterial flora. The integration of activated silica solution as a topical treatment, combined with broad-spectrum therapeutic agents targeting dysbiotic conditions of the vaginal microflora, has shown promise in normalizing the vaginal ecosystem and significantly reducing recurrence rates of UGC. This approach provides a minimally invasive, highly effective treatment option for vulvovaginal candidiasis, offering the potential for complete disease resolution without side effects. Additionally, it facilitates a reduction in treatment duration, eliminates relapses, and enables management on an outpatient basis, thereby improving the quality of life for affected women. Further research is needed to refine these therapeutic strategies and optimize their application in clinical practice, ultimately advancing the management of RVVC through a holistic understanding of vaginal biotope disturbances and immune modulation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML