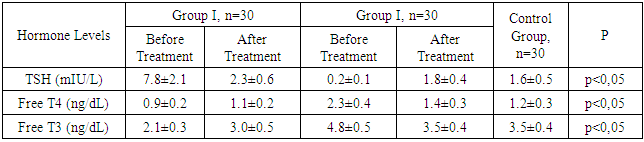-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(9): 2311-2317
doi:10.5923/j.ajmms.20241409.40
Received: Aug. 9, 2024; Accepted: Sep. 7, 2024; Published: Sep. 21, 2024

The Role of Thyroid Dysfunction in the Development of Endocrine Infertility in Women and Modern Treatment Methods
Anvarova Sh. A.1, Shukurov F. I.2
1Assistant of the Department of Obstetrics and Gynecology, Tashkent Medical Academy, Tashkent, Uzbekistan
2Doctor of Medical Sciences, Head of the Department of Obstetrics and Gynecology, Tashkent Medical Academy, Uzbekistan
Correspondence to: Shukurov F. I., Doctor of Medical Sciences, Head of the Department of Obstetrics and Gynecology, Tashkent Medical Academy, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Aim. To assess the role of thyroid dysfunction in the development of endocrine infertility in women and to develop modern treatment methods aimed at restoring reproductive function. Materials and methods. The study included 90 women with endocrine infertility caused by thyroid dysfunction. The patients were divided into two main groups based on the form of thyroid dysfunction: Group I (hypothyroidism) included 30 women with infertility due to hypothyroidism; Group II (hyperthyroidism) included 30 women with infertility due to hyperthyroidism. The control group consisted of 30 healthy women of reproductive age. All patients underwent clinical examination, which included medical history and general physical examination with a focus on symptoms of thyroid dysfunction, laboratory tests including serum levels of thyroid-stimulating hormone (TSH), free thyroxine (T4), free triiodothyronine (T3) by enzyme-linked immunosorbent assay (ELISA), antibodies to thyroid peroxidase (TPO-Ab) and thyroglobulin. Results. Hormonal study results before treatment showed that in Group I, the average TSH level was 7.8±2.1 mIU/L, significantly higher than in the control group (1.6±0.5 mIU/L, p<0.01). The average free T4 level was reduced to 0.9±0.2 ng/dL compared to the control group (1.2±0.3 ng/dL, p<0.05). TPO-Ab levels were elevated in 60% of patients in Group I. In Group II, the average free T4 level was increased to 2.3±0.4 ng/dL, significantly higher than in the control group (p<0.01). The TSH level was reduced to 0.2±0.1 mIU/L (p<0.01). TPO-Ab levels were elevated in 50% of patients in Group II. Hormonal study results after 6 months of therapy with Tyromine in patients with hypothyroidism (Group I) showed a significant decrease in TSH levels to 2.3±0.6 mIU/L (p<0.01) and an increase in free T4 levels to 1.1±0.2 ng/dL (p<0.05). Regular menstrual function was restored in 84% of patients, and 83.4% achieved pregnancy. In the group of patients with hyperthyroidism (Group II), Tyromine therapy led to a decrease in free T4 levels to 1.4±0.3 ng/dL (p<0.01) and an increase in TSH levels to 1.8±0.4 mIU/L (p<0.01). Menstrual cycles normalized in 85% of patients, and 84.5% achieved pregnancy. Conclusion. Our study demonstrated that thyroid dysfunction plays a significant role in the development of endocrine infertility in women, significantly impacting their reproductive function. Hypothyroidism and hyperthyroidism lead to severe hormonal imbalances, adversely affecting the menstrual cycle, ovulation, and overall fertility. The use of Tyromine showed high effectiveness in normalizing thyroid hormone levels, improving clinical outcomes, and restoring reproductive function in 84.5% of patients with thyroid dysfunction.
Keywords: Hypothyroidism, Hyperthyroidism, Female endocrine infertility, Thyroid hormones, Tyromine
Cite this paper: Anvarova Sh. A., Shukurov F. I., The Role of Thyroid Dysfunction in the Development of Endocrine Infertility in Women and Modern Treatment Methods, American Journal of Medicine and Medical Sciences, Vol. 14 No. 9, 2024, pp. 2311-2317. doi: 10.5923/j.ajmms.20241409.40.
1. Introduction
- Endocrine infertility is one of the key issues in women's reproductive health, affecting approximately 30-40% of all infertility cases [1,2]. Among the various endocrine disorders, thyroid dysfunction holds a special place, significantly impacting reproductive function [3,4]. Thyroid hormones play a crucial role in regulating the menstrual cycle, ovulation, and the overall condition of the reproductive system [5,6]. An imbalance of these hormones, caused by conditions such as hypothyroidism and hyperthyroidism, can lead to severe fertility issues [7,8].Hypothyroidism is characterized by insufficient production of thyroid hormones, resulting in elevated thyroid-stimulating hormone (TSH) levels and reduced thyroxine (T4) levels. This condition is often associated with ovulatory disorders, amenorrhea, and oligomenorrhea [9,10]. On the other hand, hyperthyroidism, caused by excessive production of thyroid hormones, can also negatively affect reproductive function, causing changes in TSH and thyroxine levels, leading to irregular menstrual cycles and other reproductive problems [11,12].Modern advances in medicine offer new approaches to diagnosing and treating thyroid dysfunction in women with endocrine infertility. Targeted diagnostic methods, including genetic and molecular studies, allow for more precise identification of the causes of endocrine disorders [13,14]. These methods include the use of advanced technologies such as next-generation sequencing (NGS), which can detect even minimal genomic changes associated with thyroid dysfunction. Additionally, new biomarkers are being actively developed for early diagnosis and monitoring of thyroid diseases [15,16].The need for this study is driven by the increasing prevalence of endocrine infertility caused by thyroid dysfunction and the necessity to develop more effective and individualized treatment methods. Developing such methods requires a comprehensive understanding of the mechanisms underlying thyroid dysfunction and its impact on reproductive function [17,18]. It is also important to consider a multidisciplinary approach to treatment, involving endocrinologists, gynecologists, reproductive specialists, and geneticists [19,20]. This approach will not only improve treatment outcomes but also enhance the quality of life for women suffering from endocrine infertility. The importance of this approach is underscored by the fact that successful treatment of thyroid dysfunction can significantly increase the chances of successful conception and pregnancy. Thus, this study aims to delve into the role of thyroid dysfunction in the development of endocrine infertility in women and to seek modern treatment methods that effectively address this issue.The objective of this study is to assess the role of thyroid dysfunction in the development of endocrine infertility in women and to develop modern treatment methods aimed at restoring reproductive function.
2. Materials and Methods
- The study is a clinical, prospective, case-control study. Ninety women with endocrine infertility caused by thyroid dysfunction were included in the study. The patients were divided into two main groups depending on the form of thyroid dysfunction: Group I included 30 women with infertility caused by hypothyroidism; Group II included 30 women with infertility caused by hyperthyroidism. The control group consisted of 30 healthy women of reproductive age.Inclusion Criteria: Women aged 20 to 40 years; diagnosis of endocrine infertility caused by thyroid dysfunction (hypothyroidism or hyperthyroidism), confirmed clinically and through laboratory tests; absence of other causes of infertility (tubal, uterine, male factors); confirmed irregular menstrual function; informed consent to participate in the study.Exclusion Criteria: Presence of other endocrine diseases, such as diabetes, polycystic ovary syndrome; uterine and tubal anomalies confirmed by hysterosalpingography or laparoscopy; presence of severe somatic diseases (cardiovascular, renal, hepatic, etc.); use of hormonal therapy in the last 6 months before inclusion in the study.Methods for diagnosing thyroid dysfunction and assessing reproductive function included: Clinical examination, including medical history (duration of infertility, previous pregnancies, presence of comorbidities) and general physical examination with a focus on symptoms of thyroid dysfunction (weight changes, skin changes, pulse, etc.); laboratory tests, including serum levels of thyroid-stimulating hormone (TSH), free thyroxine (T4), free triiodothyronine (T3) by enzyme-linked immunosorbent assay (ELISA), antibodies to thyroid peroxidase (TPO-Ab) and thyroglobulin (TG-Ab) to assess the autoimmune component, as well as hormonal profile assessment: levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol, and progesterone; instrumental studies, including ultrasound examination (US) of the thyroid gland to assess its structure and size, as well as pelvic ultrasound to assess the condition of the ovaries, endometrial thickness, and presence of ovulation.Patients with thyroid dysfunction were prescribed Tyromine, with the dosage adjusted depending on the form of dysfunction: for Group I, the initial dose of Tyromine was 50 µg/day with gradual increase until euthyroid status was achieved, TSH levels were monitored every 6-8 weeks to adjust the dose; for Group II, Tyromine was prescribed at a dose of 5-10 mg/day to reduce thyroid hormone levels, free T4 and T3 levels were monitored every 4-6 weeks to adjust the dose. Patients regularly underwent follow-up examinations every 3 months to assess treatment efficacy and its impact on reproductive function. Treatment efficacy was evaluated by the dynamics of hormonal indicators, restoration of menstrual function, and achievement of pregnancy.For statistical data processing, descriptive statistics methods and parametric tests were used. Continuous variables were expressed as mean and standard deviation, while categorical variables were expressed as frequencies and percentages. Group comparisons were made using the Student's t-test for independent samples and the χ² test for categorical data analysis. Differences were considered statistically significant at p<0.05. Data analysis was performed using SPSS software version 22.0.
3. Study Results
- The study included 90 women with endocrine infertility caused by thyroid dysfunction. The patients were divided into two main groups depending on the form of thyroid dysfunction: Group I included 30 women with infertility caused by hypothyroidism; Group II included 30 women with infertility caused by hyperthyroidism. The control group consisted of 30 healthy women of reproductive age. The mean age of the patients in the groups did not have statistically significant differences (p>0.05) and was 32.5±4.2 years for Group I, 31.8±3.9 years for Group II, and 31.6±4.1 years for the control group. The average duration of infertility was also similar between the groups, being 4.2±1.3 years for Group I and 3.9±1.2 years for Group II (p>0.05).To determine the role of thyroid dysfunction in the development of endocrine infertility in women and to develop modern treatment methods aimed at restoring reproductive function, we conducted hormonal and ultrasound examinations of the patients. The results of the hormonal study showed that in Group I, the average TSH level was 7.8±2.1 mIU/L, which was significantly higher than in the control group (1.6±0.5 mIU/L, p<0.01). This indicates pronounced hypothyroidism in the patients of this group. The average free T4 level was reduced to 0.9±0.2 ng/dL, indicating insufficient thyroxine production compared to the control group (1.2±0.3 ng/dL, p<0.05). The average free T3 level was also significantly reduced to 2.1±0.3 ng/dL compared to the control group (3.5±0.4 ng/dL, p<0.01), confirming the presence of severe triiodothyronine deficiency in women with hypothyroidism. The TPO-Ab level was elevated in 60% of patients in Group I, with an average value of 150±30 IU/mL.In Group II, the results showed that the average free T4 level was significantly increased to 2.3±0.4 ng/dL, which is above normal and significantly different from the control group (1.2±0.3 ng/dL, p<0.01), indicating hyperthyroidism. The TSH level was significantly reduced to 0.2±0.1 mIU/L (compared to 1.6±0.5 mIU/L in the control group, p<0.01), characteristic of increased thyroid activity and autonomy. The average free T3 level was increased to 4.8±0.5 ng/dL, which was also significantly higher than the control values (3.5±0.4 ng/dL, p<0.01) and confirms the diagnosis of hyperthyroidism. The TPO-Ab level was elevated in 50% of patients in Group II, with an average value of 120±25 IU/mL (see Fig. 1).
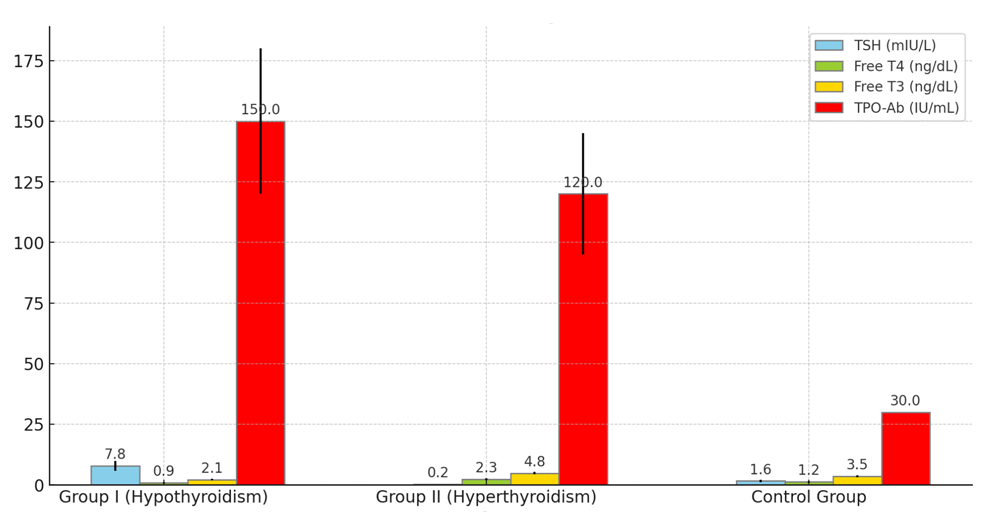 | Figure 1. Average Hormone Levels in Women with Thyroid Dysfunction |
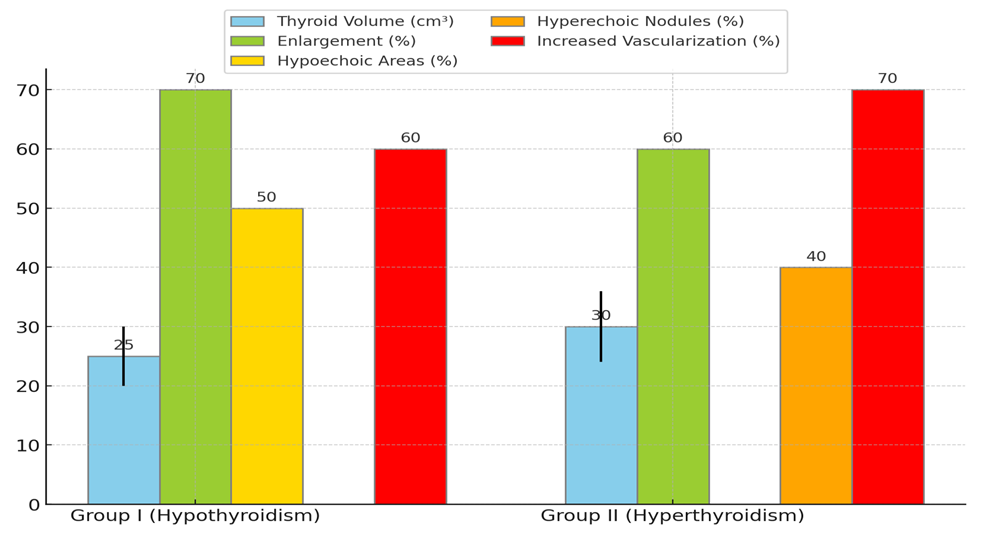 | Figure 2. Echodopplerographic Indicators of the Thyroid Gland in Women with Thyroid Dysfunction |
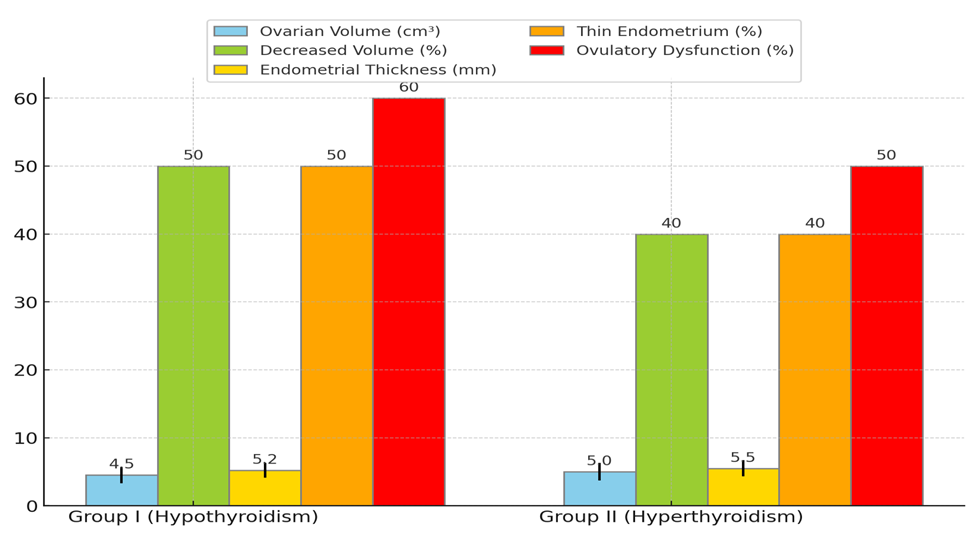 | Figure 3. Echographic Indicators of the Uterus and Adnexa in Women with Thyroid Dysfunction |
|
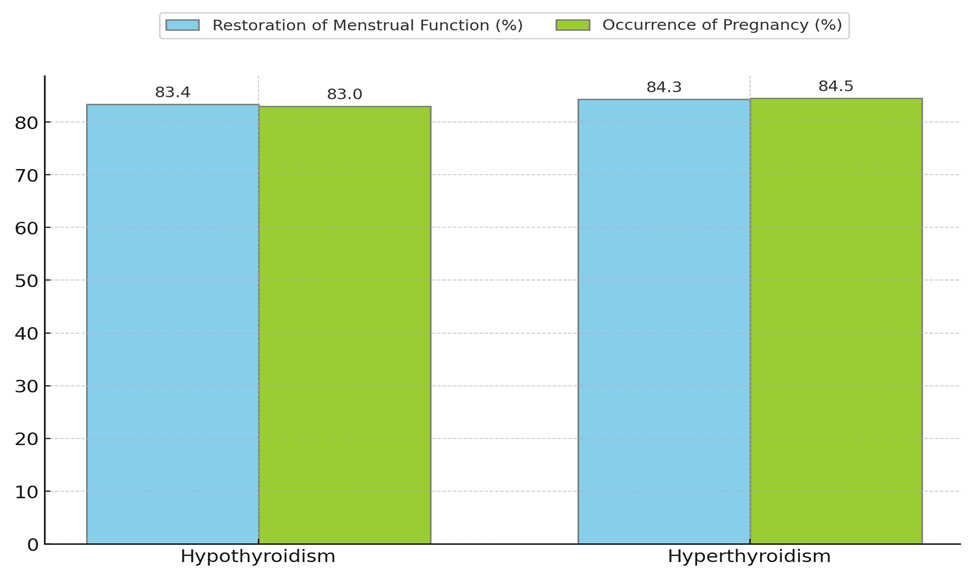 | Figure 4. Effectiveness of Tyramine Treatment in Studied Women |
4. Discussion
- In this study, clinical, laboratory, and instrumental indicators were assessed in women with endocrine infertility caused by thyroid dysfunction. The main objective was to identify the impact of hypothyroidism and hyperthyroidism on reproductive function and evaluate the effectiveness of Tyramine treatment.The results of the hormonal study before treatment showed significant changes in thyroid hormone levels in patients with hypothyroidism and hyperthyroidism. Women with hypothyroidism had significantly elevated TSH levels and reduced free T4 and T3 levels, indicating insufficient thyroid function. The high TPO-Ab level in most patients suggests a possible autoimmune nature of the disease. Women with hyperthyroidism showed the opposite pattern: high levels of free T4 and T3, and low TSH levels, indicating hyperthyroid function. The elevated TPO-Ab level also indicates a possible autoimmune origin of the disease.Ultrasound examination of the thyroid gland before treatment revealed an increase in its size in most patients with thyroid dysfunction, which is associated with compensatory and inflammatory processes. These changes included structural heterogeneity and vascularization changes, confirming the presence of an active pathological process in the gland.Instrumental studies of the pelvic organs before treatment showed the negative impact of thyroid dysfunction on the reproductive system. Patients with hypothyroidism and hyperthyroidism had reduced ovarian volume and endometrial thickness, which is a significant factor in the development of endocrine infertility. Ovulatory dysfunctions, manifested by the absence of dominant follicles and cyst formation, were also identified in a significant number of patients.Tyramine therapy proved effective in normalizing thyroid hormone and thyroid peroxidase antibody levels. After 3 months of treatment, patients with hypothyroidism showed a significant decrease in TSH levels and an increase in free T4 and T3 levels. The TPO-Ab level also decreased, indicating a reduction in autoimmune inflammation. Patients with hyperthyroidism showed a decrease in free T4 and T3 levels and an increase in TSH levels, indicating normalization of thyroid function. The TPO-Ab level also decreased, confirming a reduction in the autoimmune process.Ultrasound studies after treatment showed positive changes in the structure and functionality of the thyroid gland and pelvic organs. The reduction in thyroid gland size, improvement in its structure, decreased vascularization, and increased ovarian volume and endometrial thickness confirm the effectiveness of the therapy. Most patients restored regular menstrual function, and a significant number of women achieved pregnancy, indicating the restoration of reproductive function.Thus, this study confirms the significant role of thyroid dysfunction in the development of endocrine infertility in women. Tyramine treatment has shown high effectiveness in normalizing hormonal profiles and improving reproductive function in patients with hypothyroidism and hyperthyroidism. These data underscore the necessity of timely diagnosis and treatment of thyroid dysfunction to improve women's reproductive health.
5. Conclusions
- Our study has shown that thyroid dysfunction plays an important role in the development of endocrine infertility in women, significantly impacting their reproductive function. Hypothyroidism and hyperthyroidism lead to serious hormonal imbalances, adversely affecting the menstrual cycle, ovulation, and overall fertility. The use of Tyramine has demonstrated high effectiveness in normalizing thyroid hormone levels, improving clinical outcomes, and restoring reproductive function in 84.5% of patients with thyroid dysfunction. Tyramine treatment led to significant improvement in hormonal indicators in women with hypothyroidism and hyperthyroidism, as evidenced by decreased TSH levels and normalization of free T4 levels. In patients with hypothyroidism, Tyramine treatment contributed to the restoration of regular menstrual function in 83.4% and pregnancy in 83%, while in patients with hyperthyroidism, the menstrual cycle normalized in 84.3% and pregnancy occurred in 84.5% of women.Overall, our study highlights the importance of early diagnosis and timely correction of thyroid dysfunction to improve women's reproductive health. The use of modern treatment methods, including Tyramine, provides effective means for restoring hormonal balance and reproductive function in women with endocrine infertility. These findings confirm the need for further research in this area to develop more effective and individualized treatment methods.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML