-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(9): 2303-2306
doi:10.5923/j.ajmms.20241409.38
Received: Sep. 3, 2024; Accepted: Sep. 17, 2024; Published: Sep. 20, 2024

Program for Predicting the Development of Benign Breast Diseases in Women with Hyperplastic Processes of the Uterus
Askarova Zebo Zafarjonovna1, Kurbaniyazova Madina Zafarjanovna2
1Samarkand State Medical University, Samarkand, Republic of Uzbekistan
2Urgench Branch of Tashkent Medical Academy, Urgench, Republic of Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The study included the results of 82 patients with uterine hyperplastic processes (HPPU), who underwent a molecular genetic examination to determine the frequency of distribution of alleles and genotypes of the rs1138272 polymorphism in the GSTP1 gene (Ile/Val) in patients with HPPU, patients with HPPU and BMD, and conditionally -healthy female donors. Currently, an important direction of modern research in the field of preventive medicine is to determine the risk of developing pathology based on the search for significant molecular genetic predictors, and this method also makes it possible to determine the predisposition to hyperplastic processes of the uterus and mammary glands, their relapses and the development of neoplasia.
Keywords: Polymorphism gene, Uterine hyperplastic processes
Cite this paper: Askarova Zebo Zafarjonovna, Kurbaniyazova Madina Zafarjanovna, Program for Predicting the Development of Benign Breast Diseases in Women with Hyperplastic Processes of the Uterus, American Journal of Medicine and Medical Sciences, Vol. 14 No. 9, 2024, pp. 2303-2306. doi: 10.5923/j.ajmms.20241409.38.
1. Introduction
- In recent years, the incidence of benign breast dysplasia (BMD) in patients with gynecological pathology has increased worldwide. Hyperplastic processes of the uterus (HPPU) and mammary glands are one of the most common diseases in gynecological clinics and in our Republic.According to the literature, HPPU can be detected in women aged 20-30, and sometimes even earlier. However, after 40 years, the risk of HPPU increases to 76-80%, and the disease continues to be a fairly common pathology in women during the perimenopause period.GST genetic polymorphism is the main reason for many neurological dysfunctions. GST has over expressed in epileptic brain and pi (π) GST has used to predict stroke; mu (μ) and pi (π) GST are over expressed in Alzheimer's disease (AD).HPPU is often the only pathology of the reproductive system, but it is often accompanied by hyperplastic processes of the mammary glands, which is explained by the same type of neuroendocrine changes in the hypothalamic-pituitary-ovarian and adrenal systems. Research conducted in recent years indicates a common pathogenesis of mammary gland pathology and dyshormonal diseases of the genital organs. According to G.M. Savelyeva, the development of uterine fibroids and mastopathy occurs simultaneously and the probability of a combination of these processes is 76-87%. According to some authors, the most severe forms of BMD are formed in women with uterine fibroids, adenomyosis, and hyperplastic processes of the endometrium. Other researchers suggest considering the priority of damage to the mammary glands as a marker of developing unified disorders in the reproductive system.The concept of the multifactorial nature of hyperplastic diseases of the female reproductive system that has emerged in recent years suggests a special involvement of endocrine, immunological, hormonal, environmental, and genetic factors, the relative role of which is different in the genesis of each disease.Despite a large number of studies on the condition of the mammary glands in terms of gynecological practice, many issues related to the principles of treatment and management of patients with combined hyperplasia of two hormone-dependent structures, methods for preventing the development of cancer and severe forms of mastopathy in patients with endometrial hyperplastic processes during perimenopause have not been sufficiently covered.Research objective: To optimize the management of women with uterine hyperplastic processes by determining the polymorphism of the GSTP1 gene.GSTP1 encodes an enzyme involved in Phase II drug metabolism that catalyzes the conjugation of glutathione to xenobiotics, leading to their excretion. [3]
2. Research Materials and Methods
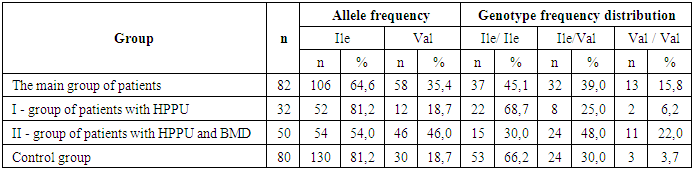
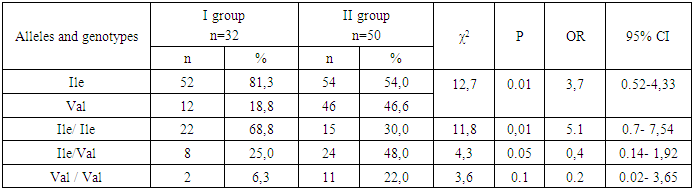
- To analyze the distribution of allele and genotype frequencies of the rs1138272 polymorphisms in the GSTP1 gene (Ile/Val) in the study groups, their distribution by the studied polymorphic loci was checked for compliance with the RHC using Fisher's exact test in a sample group of 80 conditionally healthy female donors (control group) and 82 patients with HPPU (Group I - patients with HPPU (n=32) and Group II - patients with HPPU who had BMD (n=50)). Our analysis of the distribution of allele and genotype frequencies of the rs1138272 polymorphism in the GSTP1 gene (Ile/Val) in the group of conditionally healthy donors (control) allowed us to establish the following facts: the frequency of the Ile allele was 81.2%, and the Val allele - 18.7% of the case. At the same time, carriage of the homozygous genotype Ile/Ile was determined in 66.2% (n=53), heterozygous genotype (Ile/Val) in 30.0% (n=24), and homozygous Val/Val in 3.7% (n=3) of cases (Table 1).
|
|
|
|
3. Conclusions
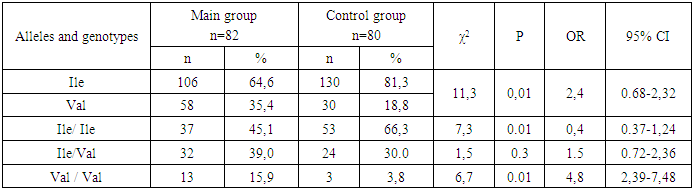
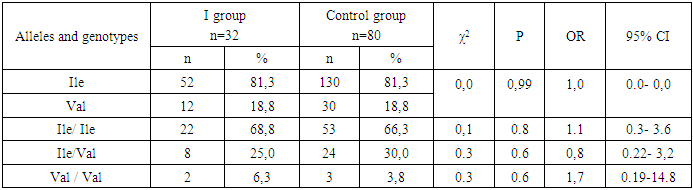
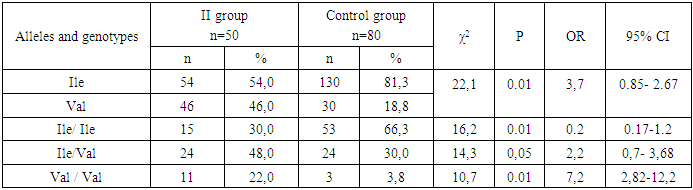
- Thus, the obtained data indicate the presence of statistically significant differences in the distribution of the frequencies of the Ile and Val alleles of the mutant genotypes Ile/Ile and Val/Val of the rs1138272 polymorphism in the GSTP1 gene (Ile/Val) between the main group of patients with uterine hyperplastic processes and conditionally healthy donors, which in turn allows us to identify these alleles and genotypes as genetic factors predisposing to an increased risk of developing [4] uterine hyperplastic processes and benign breast dysplasia in women during perimenopause. Statistically significant differences were established in comparison with the values in conditionally healthy donors. In particular, in the main group of patients, an increase in the frequency of the Val allele by almost two times and the homozygous genotype and Val/Val by 2.4 times were established due to their levels in the group of patients with HPPU and BMD. These facts prove the role of the Val allele and the heterozygous genotype Ile / Val polymorphism rs1138272 in the GSTP1 gene (Ile / Val) in the risk of developing hyperplastic processes of the uterus and especially the combination of this pathology with benign dysplasia of the mammary glands in women in menopause.
References
| [1] | Novikova O.V. A new version of hormonal treatment of atypical hyperplasia and early endometrial cancer with preservation of fertility / O.V. Novikova, Ch.A. Avasova, E.G. Novikova et al. // Oncogynecology. - 2019. - No. 1 - P. 36-45. |
| [2] | Radzinsky, V.E. Medicine of the mammary gland and gynecological diseases. M.: Status Praesens; 2017. 335 p. |
| [3] | Strizhakov A.N. et al. Differentiated approach to diagnostics and tactics of management of patients with hyperplastic processes of the endometrium in postmenopause // Issues of gynecology, obstetrics and perinatology. - 2014. - No. 1. - P. 5-14. |
| [4] | Todjieva N.I., Khudoyarova D.R., Bazarova Z.Z. Improving methods for treating endometrial hyperplastic processes in premenopause - Professional of the Year. 2018. 81-84 p. |
| [5] | Yuldasheva D.Yu., Karimov H.Ya., Boboev A.T., Komilova I.A., Sadikova D.R. Method for predicting cervical neoplasia in women with endometrial hyperplastic processes // IAP 2015 0492 Uzbekistan Intellectual Property League 01/29/2016. - No. 1 (177). — P. 38. 6. Asaturova A., Dumanovskaya M., Chernukha G., Kogan E., Fayzullina N. The innovative approach to PI3/AKT-signalling pathway unbalance in endometrial hyperplasia and it's modulation with micronized progesterone // Abstracts of the 20th World Congress on Controversies in Obstetrics, Gynecology & Infertility (COGI) (Paris, December 04-07, 2014) // Abstract book - 2014. - P. 44. 7. Cipora E., Konieczny M., Sobieszczanski J. Acceptance of illness by women with breast cancer. Annals of Agricultural and Environmental Medicine. 2018; 25 (1): 167-171. |
| [6] | Cui, J., Li, G., Yin, J., Li, L., Tan, Y., Wei, H., ... & Yi, L. (2020). GSTP1 and cancer: Expression, methylation, polymorphisms and signaling. International journal of oncology, 56(4), 867-878. |
| [7] | Ishii, T., Matsuse, T., Teramoto, S., Matsui, H., Miyao, M., Hosoi, T., ... & Ouchi, Y. (1999). Glutathione S-transferase P1 (GSTP1) polymorphism in patients with chronic obstructive pulmonary disease. Thorax, 54(8), 693-696. |
| [8] | Li, D., Dandara, C., & Parker, M. I. (2010). The 341C/T polymorphism in the GSTP1 gene is associated with increased risk of oesophageal cancer. BMC genetics, 11, 1-9. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML