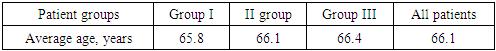-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(9): 2256-2261
doi:10.5923/j.ajmms.20241409.28
Received: Aug. 23, 2024; Accepted: Sep. 17, 2024; Published: Sep. 18, 2024

Clinical-Neurological and Biochemical Parallels in Frontotemporal Dementia
Rakhimbaeva Gulnora Sattarovna1, Abramyan Arevik Armikovna2, Nasirdinova Nargisa Askarovna2
1Tashkent Medical Academy, Tashkent, Uzbekistan
2Andijan State Medical Institute, Andijan, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Frontotemporal dementia (FTD) is a group of neurodegenerative diseases with predominant damage to the frontal and anterior temporal lobes of the brain, manifested by progressive behavioral and personality disorders with a gradual loss of empathy and the ability to productive contact, as well as cognitive impairment. Various authors have put forward theories about the influence of the level of neurohormones on the development of cognitive impairment in degenerative diseases of the brain. The study showed that cognitive impairment is not the last in the clinic of frontotemporal dementia, and the severity of cognitive impairment has an inverse correlation with the level of the hormone cortisol and a direct correlation with the level of the hormone dehydroepiandrosterone sulfate. Having identified the mechanism of influence of these hormones, it is possible to prevent cognitive impairment, as well as delay pronounced cognitive deficit, by correcting their level, as well as by influencing the production of these hormones in the body itself.
Keywords: Frontotemporal dementia, Cortisol, Dehydroepiandrosterone sulfate, Cognitive impairment
Cite this paper: Rakhimbaeva Gulnora Sattarovna, Abramyan Arevik Armikovna, Nasirdinova Nargisa Askarovna, Clinical-Neurological and Biochemical Parallels in Frontotemporal Dementia, American Journal of Medicine and Medical Sciences, Vol. 14 No. 9, 2024, pp. 2256-2261. doi: 10.5923/j.ajmms.20241409.28.
Article Outline
1. Introduction
- Dementia is a syndrome characterized by disturbances in the mnemonic and other cognitive areas, including speech, orientation, abstract thinking, and praxis. The main causes of dementia are degenerative processes (Alzheimer's disease - (50-60%), vascular pathology - (10-15%), a combination of degenerative and vascular processes (10-20%) [1,12]. FTD accounts for 5-7% of dementia cases. FTD is second only to Alzheimer's disease among cases of presenile dementia and accounts for 10-20% of all dementias in this age group [15]. The incidence is 8.9 cases per 100 thousand people per year. The peak incidence is at the age of 55-65 years, although rare cases of the onset of the disease at 20-40 years and even after 90 years have been described [1,33]. FTD is equally common among men and women, although according to some authors, men predominate [15].Since 1998, the diagnosis of FTD has been based on the clinical criteria proposed by Neary et al. According to these criteria, FTD was diagnosed in the presence of the main clinical manifestations:• gradual onset and steady progression;• early developing personality and social behavior disorder;• emotional indifference;• reduction of criticism.There are 2 large groups of frontotemporal dementia: the behavioral form of FTD and primary progressive aphasia (PPA). Primary progressive aphasia, in turn, is subdivided into the semantic form of PPA, the agrammatic form of PPA and the logopenic form of PPA [2,5,8,9,10,11,14].The clinical manifestations of the behavioral form of frontotemporal dementia are based on damage to the frontal lobes of the brain, due to which this form manifests itself as a combination of behavioral, cognitive, and affective disorders. The personality structure and social aspects of the patient's personality suffer. Clinically, this is manifested by impulsiveness, failure to observe hygiene rules, antisocial behavior, decreased concentration, mental rigidity, and stereotypical behavior. Patients can endlessly sort through objects, make stereotypical hand movements, go to the same place, and are prone to theft. Many have movement disorders in the form of Parkinson's syndrome. Cognitive disorders later join in.The second type of frontotemporal dementia is primary progressive aphasia syndrome, which is characterized by progressive speech disorders that arise gradually, without apparent cause, and tend to progress continuously. In this case, cognitive impairment may be absent in the early stages [2].Speech functions are lost due to asymmetric (more left-sided) anterolateral atrophy of the temporal lobes; the hippocampus and memory are affected to a lesser extent. Patients experience difficulties in finding words when communicating, constructing sentences, and have trouble counting. There is significant variability in the time between the development of the first signs of speech disorders and the impairment of the cognitive sphere, from 10 to 2-3 years. The first clinical description and proposal of the term "primary progressive aphasia" belongs to M. Mesulam [3,27]. In accordance with the currently proposed criteria, there are 3 main forms of PPA: semantic form (SF) without a decrease in speech fluency (English - fluent aphasia, semantics dementia), agrammatical form (AF) with decreased speech fluency (English - non-fluent aphasia) and logopenic form (LF) [3,17,18,27,28,30]. The third - logopenic - variant of primary progressive aphasia in most cases is an atypical form of Alzheimer's disease, and not a manifestation of frontotemporal degeneration [4,6,13]. A combined form of PPA is also distinguished, which has signs similar to SF and AF PPA [3,29].In the agrammatic form of primary progressive aphasia, the patient's speech becomes laconic, erroneous, and intermittent; the grammatical structure is disrupted: the patient speaks in unrelated words or phrases. Pauses, verbal perseverations resembling stuttering, and literal paraphasias are characteristic of the conversation. At the same time, the patient understands the speech addressed to him [8,11,17,18,31]. A similar clinical picture is observed in Broca's aphasia. Neuroimaging of this form of primary progressive aphasia is characterized by a defect in the left frontal or frontotemporal regions of the cerebral hemispheres [17,31].The leading characteristic of speech disorders in SF PPA is the impairment of understanding the meaning and significance of words, especially nouns. They cannot name objects, cannot remember their name, explain its purpose, and replace some words with others that are close in meaning. Their own speech retains the correct grammatical structure. At the same time, patients can repeat after the doctor and read aloud, although they do not always understand the meaning of what they read. Unlike semantic memory, episodic memory for current and remote life events is not affected in SF PPA. Structural neuroimaging methods usually reveal atrophy, and functional neuroimaging methods (positron emission tomography - PET and SPECT) - hypoperfusion or hypometabolism in the anterior temporal lobe, primarily the left hemisphere [3,17,18].Clinical manifestations of the logopenic form of primary progressive aphasia are difficulties in selecting the right words in spontaneous speech and when naming, the patient has difficulty repeating a phrase or sentence after the doctor. Grammatically, speech is not impaired, the receptive component of speech is preserved, as is semantic memory [17,18]. Structural neuroimaging techniques in LF typically reveal atrophy of the parietal and posterior regions of the left temporal lobe [17,18].Although cognitive impairments in frontotemporal dementia are not the most important clinical feature of the disease, their presence significantly aggravates the course of the disease and the patient's social activity. Various authors have put forward theories about the influence of neurohormones on the development of cognitive impairments in degenerative diseases of the brain. Such hormones include corticosteroids, which affect mood, stress, anxiety, sleep, appetite, and cognitive abilities [24,32,35].Once released from the adrenal cortex, cortisol readily crosses the blood-brain barrier and binds to specific intracellular receptors in the brain, particularly in areas involved in cognitive function [26]. Once activated, these receptors bind to “hormonal response elements” in DNA and regulate the transcription of target genes [20,31,32].The effect of cortisol on cognitive abilities depends on its level in the blood and the duration of its effect on the brain. With short-term exposure to moderate doses of cortisol, for example during stress, cognitive functions improve due to the activating effect of cortisol on the mineralocorticoid receptors of the hypothalamus, which have a positive effect on cognitive functions. With an increase in the level and duration of exposure, activation of glucocorticoid receptors occurs, which have a negative effect on cognitive functions [16,23,24,31,32].Altered functioning of the hypothalamic-pituitary-adrenal axis and, in particular, high cortisol levels in older adults have been associated with an increased risk of developing dementia and Alzheimer's disease [23,24,31,32,34].Dehydroepiandrosterone (DHEA) is a hormone produced in the adrenal glands that acts on androgen receptors. DHEA is formed from pregnenolone, which in turn is formed from cholesterol. These processes occur under the action of the enzyme 17-alpha-hydroxylase in the zona reticularis of the adrenal cortex [7]. There is evidence that 70% of dehydroepiandrosterone sulfate and 85% of its less active metabolite dehydroepiandrosterone sulfate are formed in the adrenal glands and only 10% are formed in the gonads [7]. A certain portion of dehydroepiandrosterone is formed in the central nervous system, also from pregnenolone [19]. Dehydroepiandrosterone is chemically similar to testosterone and estradiol and can be easily converted into it. In early and adulthood, the maximum content of this hormone is observed, then its production decreases. Therefore, the norm for determining the metabolite of this hormone - dehydroepiandrosterone sulfate in the blood is different at different periods of life, and also differs in men and women. Dehydroepiandrosterone sulfate is most often determined for diagnostic purposes, due to the fact that it is present in the blood in high concentrations, and has a long half-life and high stability [7]. It is known that dehydroepiandrosterone sulfate has a positive effect on the cognitive sphere, emotional background, mood, memory [7]. Due to the fact that dehydroepiandrosterone sulfate can be synthesized in the brain, it was called a neurosteroid, which performs many important neurophysiological functions [7,19,25].In various studies conducted on animals, it was found that dehydroepiandrosterone and glucocorticoids have opposite effects on the brain. While corticosteroids have a toxic effect on the brain, dehydroepiandrosterone, on the contrary, has a beneficial effect [7,22]. Various authors attributed the negative signs of aging to a decrease in the level of the hormone dehydroepiandrosterone sulfate in old age. Studies were conducted in which it was found that elderly women with low levels of dehydroepiandrosterone sulfate had more pronounced depression than men, and a high cortisol/DHEA ratio correlated with a decrease in memory functions in both sexes [7,21].Further study of the relationships between neurohormones and cognitive changes in various neurodegenerative diseases of the brain, including FTD, may help to discover ways to prevent and treat cognitive complications in these diseases.
2. Materials and Methods of Research
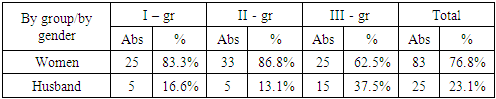
- In our study, the subject was 108 patients aged 60 to 75 years, divided into 3 groups:Group 1 consisted of 30 (27.7%) patients with FTD: of these, 19 (63.3%) patients had PF FTD, 11 (36.6%) patients had PPA.Group 2 consisted of 38 (35.1%) patients with cerebrovascular insufficiency complicated by moderate and severe cognitive impairment.The 3rd control group consisted of 4 0 (37%) patients without cognitive impairment. All patients underwent a complete neurological examination, a study using neuropsychological scales, and all groups of patients underwent a blood test for the content of the hormones cortisol and dehydroepiandrosterone sulfate.Distribution by gender showed that among 108 patients there were 83 (76.8%) women and 25 (23.1%) men. Distribution of men and women by groups showed that in the first group there were 25 (83.3%) women and 5 (16.6%) men, in the second group – 33 (86.8%) women and 5 (13.1%) men, in the third group – 25 (62.5%) women and 15 (37.5%) men (Table 1).
|
|
3. Research Results and Their Discussion
- Among the most frequently encountered clinical manifestations in patients with frontotemporal dementia, the following were identified: cognitive impairment – 100%, behavioral impairment – 100%, emotional impairment – 72.7%, speech impairment – 75.8%, sleep impairment – 36.4%, headache – 33.3%, dizziness – 24.2% (Figure 1).
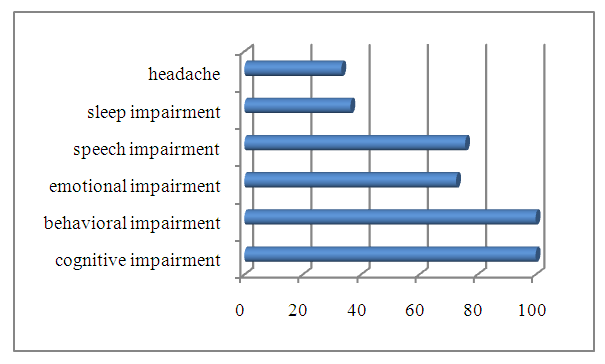 | Figure 1. The most common clinical manifestations in patients with frontotemporal dementia |
|
|
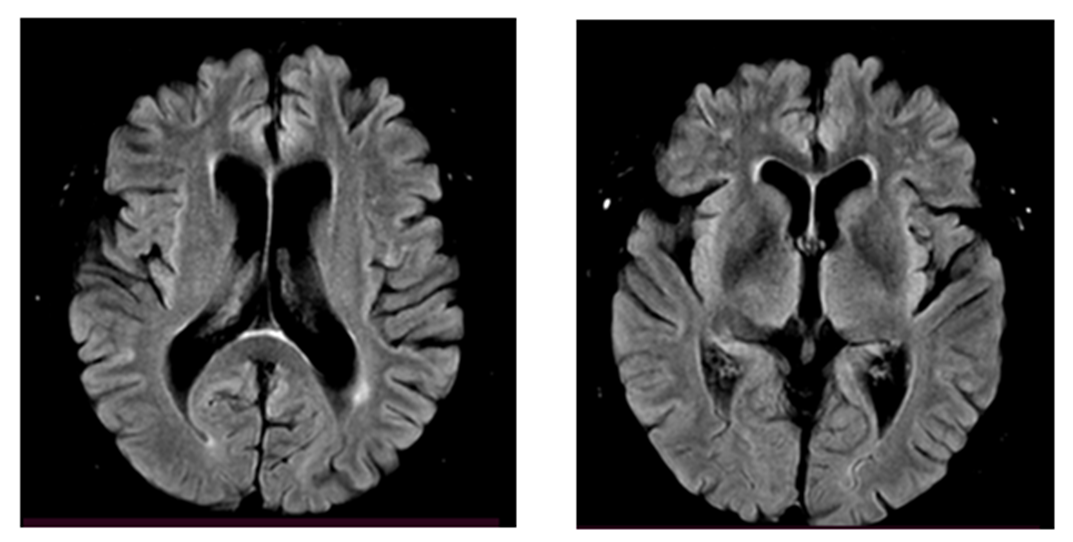 | Figure 2. MRI of patient N, 68 years old. Atrophy of the frontotemporal regions of the brain |
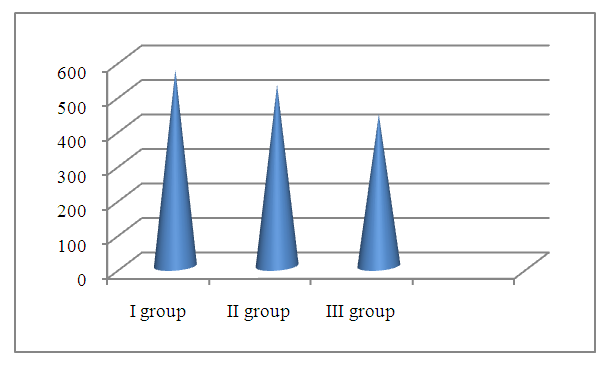 | Figure 3. Serum cortisol concentrations by groups (nmol /l) |
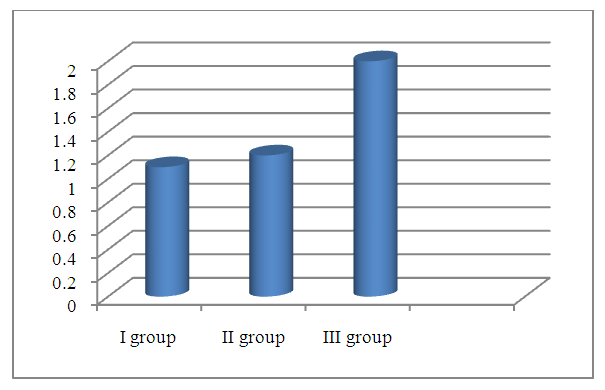 | Figure 4. Serum dehydroepiandrosterone sulfate concentrations by groups (μg/ml) |
4. Conclusions
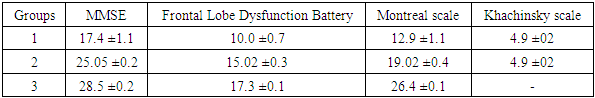
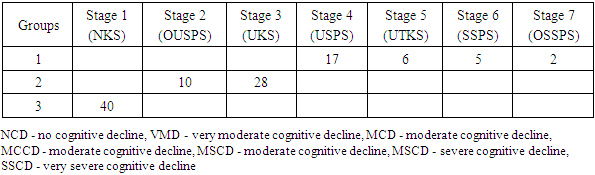
- 1. The most significant clinical manifestations of frontotemporal dementia are behavioral (aggressiveness, sloppiness, stereotypical behavior), speech (forgetting the names of objects, difficulty finding words when communicating, replacing some words with others) and emotional disorders (apathy, irritability, emotional lability, aggression), which will subsequently be joined by cognitive disorders (decreased memory, attention, abstract thinking).2. A detailed examination of patients with FTD using neuropsychological scales revealed a pronounced impairment of higher brain functions, varying from the stage of moderate cognitive decline, or early dementia in 56.6% of patients to severe or late dementia in 6.6% of patients according to the Reisberg scale, i.e. there was a wide variability of cognitive disorders. Such aspects of the cognitive sphere as memory, attention, orientation, thinking also suffer in frontotemporal dementia and these disorders play a huge role in the development of clinical manifestations of this pathology. According to all indicators of neuropsychological scales in the group with FTD, patients showed the lowest result compared to the group with moderate and severe cognitive impairment and, especially, to the control group.3. The level of the hormone cortisol in the blood of patients had an inverse correlation with the level of cognitive disorders in the examined patients, i.e. the higher the level of cortisol, the lower the cognitive level and vice versa in patients with a lower level of cortisol, cognitive functions were at a higher level, according to neuropsychological scales. This fact indicates that long-term exposure to high levels of cortisol on the brain significantly reduces cognitive activity.4. The level of the hormone dehydroepiandrosterone sulfate had a direct correlation with the level of the cognitive sphere, namely, the higher the level of this hormone, the higher the cognitive level, which was proven in a group of healthy patients without cognitive impairment, who had the highest level of DHEAS, while in patients with FTD the lowest level of this neurohormone was found.5. The introduction into practice of studying the level of the proposed neurohormones in the blood serum for the diagnosis of cognitive deficit will allow for early diagnosis of cognitive impairment and FTD, and, consequently, timely initiation of treatment and preventive measures to prevent the development of severe forms of dementia, disability and death of patients.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML