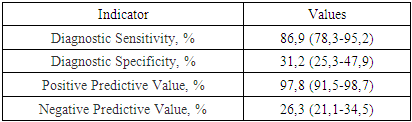-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(9): 2195-2201
doi:10.5923/j.ajmms.20241409.14
Received: Aug. 12, 2024; Accepted: Sep. 7, 2024; Published: Sep. 10, 2024

The Significance and Prognostic Role of Platelet Microvesicles in the Development of Thrombotic Complications in Patients with Cardiac Rhythm Disorders
Turayev Kh. N.1, Ziyadullayev Sh. Kh.2, Ibragimov Kh. I.1
1Samarkand State Medical University, Samarkand, Uzbekistan
2Institute of Immunology and Human Genomics of the Academy of Sciences, Tashkent, Uzbekistan
Correspondence to: Turayev Kh. N., Samarkand State Medical University, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Cardiac rhythm disorders, particularly atrial fibrillation (AF), are associated with a heightened risk of thromboembolic events, making effective management crucial for patient outcomes. This study investigates the role of platelet microvesicles (PMVs) in the development of thrombotic complications in patients with cardiac rhythm disorders, specifically those with ischemic myocardial damage. PMVs, which are small, phospholipid-enriched extracellular vesicles, have been identified as significant contributors to the hypercoagulable state observed in these patients. The study involved the quantification and characterization of PMVs in different patient groups and assessed their correlation with platelet aggregation and the incidence of thrombotic events. The results demonstrate that elevated levels of PMVs are strongly associated with increased thrombotic risk, particularly due to the presence of procoagulant factors such as phosphatidylserine (PS) and tissue factor (TF) on their surfaces. These findings suggest that PMV levels could serve as a prognostic biomarker for thrombotic complications, offering a potential tool for identifying high-risk patients who may benefit from intensified antithrombotic therapy. The study concludes that while PMVs hold promise as both biomarkers and therapeutic targets, further research is necessary to establish standardized thresholds for PMV levels and to develop targeted therapies that mitigate their procoagulant effects without disrupting physiological processes.
Keywords: Platelet Microvesicles, Cardiac Rhythm Disorders, Thrombotic Complications, Atrial Fibrillation, Ischemic Myocardial Damage
Cite this paper: Turayev Kh. N., Ziyadullayev Sh. Kh., Ibragimov Kh. I., The Significance and Prognostic Role of Platelet Microvesicles in the Development of Thrombotic Complications in Patients with Cardiac Rhythm Disorders, American Journal of Medicine and Medical Sciences, Vol. 14 No. 9, 2024, pp. 2195-2201. doi: 10.5923/j.ajmms.20241409.14.
1. Introduction
- Cardiac rhythm disorders, particularly atrial fibrillation, are among the most common cardiovascular conditions and are associated with a significantly increased risk of thromboembolic events. The pathophysiology of thrombosis in these patients is complex and multifactorial, involving endothelial dysfunction, blood stasis, and hypercoagulability. Recently, attention has been focused on the role of platelet microvesicles (PMVs) as critical contributors to the thrombotic risk in patients with cardiac rhythm disorders [1,3,6,8].Platelet microvesicles are small, phospholipid-enriched extracellular vesicles, typically ranging from 100 to 1000 nm in diameter. They are released from platelets upon activation, apoptosis, or during physiological cell aging. The biogenesis of PMVs involves the outward budding and fission of the plasma membrane, a process often triggered by increased intracellular calcium levels and cytoskeletal rearrangements [2,4,9].The surface of PMVs is enriched with procoagulant phospholipids, particularly phosphatidylserine (PS), which is normally confined to the inner leaflet of the plasma membrane but becomes externalized during vesicle formation. PMVs also carry a variety of bioactive molecules, including coagulation factors, adhesion molecules, and receptors, that facilitate their involvement in hemostasis and thrombosis [2,5,7].The procoagulant potential of PMVs is largely attributed to their surface expression of PS and tissue factor (TF). PS provides a negatively charged surface that supports the assembly of coagulation factor complexes, particularly the tenase and prothrombinase complexes, leading to the rapid generation of thrombin. Thrombin, in turn, converts fibrinogen to fibrin, culminating in clot formation [6,10].Additionally, some PMVs express TF, a potent initiator of the extrinsic coagulation pathway. TF-bearing PMVs can activate the coagulation cascade directly, leading to the amplification of thrombin generation and subsequent fibrin formation. This dual mechanism of action makes PMVs highly effective at promoting thrombosis, particularly in a hypercoagulable state such as that seen in cardiac rhythm disorders [3,6,11].Several studies have demonstrated elevated levels of PMVs in patients with cardiac rhythm disorders, especially those with atrial fibrillation. These elevated PMV levels have been correlated with markers of hypercoagulability, including increased thrombin generation and fibrin formation, as well as with clinical outcomes such as stroke and systemic embolism [5,12,13].Research has also shown that the concentration of PMVs is significantly higher in patients with cardiac rhythm disorders who develop thrombotic complications compared to those who do not. This suggests that PMV levels could serve as a biomarker for thrombotic risk in these patients. Furthermore, the presence of TF-positive PMVs in the peripheral blood of these patients has been associated with an increased risk of thromboembolism, providing further evidence of the prothrombotic role of PMVs in this population [14-17].The prognostic significance of PMVs in cardiac rhythm disorders is supported by studies showing that higher levels of PMVs are associated with an increased risk of stroke and myocardial infarction. The positive predictive value of elevated PMV levels for the development of thrombotic complications has been reported to be high, indicating that patients with increased PMV concentrations are at substantial risk for adverse thrombotic events [4,8,18,21].The use of PMV levels as a prognostic tool could help identify high-risk patients who may benefit from more aggressive antithrombotic therapy. However, further research is needed to establish standardized thresholds for PMV levels and to determine the optimal therapeutic strategies for patients with elevated PMV levels [19,20].The mechanisms by which PMVs contribute to thrombosis in cardiac rhythm disorders are multifaceted. PMVs can enhance platelet aggregation, facilitate the assembly of coagulation factor complexes, and promote the formation of a stable fibrin clot. Additionally, PMVs can interact with endothelial cells and leukocytes, further amplifying the prothrombotic state [19,22-24].In atrial fibrillation, for example, the irregular and often rapid heart rate can lead to blood stasis in the atria, which is a key factor in thrombus formation. The presence of PMVs in this context can exacerbate the prothrombotic environment by providing a surface for coagulation factor activation and by promoting platelet aggregation, thereby increasing the risk of thrombus formation and subsequent embolization [7,16,25].Given the role of PMVs in thrombosis, targeting PMVs or their procoagulant activity could be a potential therapeutic strategy for preventing thrombotic complications in patients with cardiac rhythm disorders. Antithrombotic therapies, such as anticoagulants and antiplatelet agents, may reduce the release of PMVs or inhibit their procoagulant effects, thereby lowering the risk of thrombosis [23,26].There is also interest in the development of therapies that specifically target the components of PMVs, such as PS or TF, to disrupt their procoagulant activity. However, the challenge lies in selectively targeting pathological PMVs without affecting their physiological roles in hemostasis and immune regulation [9,14].Platelet microvesicles are emerging as important players in the pathophysiology of thrombotic complications in patients with cardiac rhythm disorders. Their ability to promote coagulation through the expression of procoagulant phospholipids and tissue factor, along with their elevated levels in these patients, underscores their significance as both biomarkers and potential therapeutic targets. Future research should focus on elucidating the precise mechanisms by which PMVs contribute to thrombosis in cardiac rhythm disorders and on developing targeted therapies to mitigate this risk [2,7,20].In this context, our study aims to evaluate the role of platelet microvesicles (PMVs) in the development of thrombotic complications in patients with cardiac rhythm disorders, particularly those associated with ischemic heart disease. Specifically, the objectives of the study include quantifying and characterizing PMVs in different patient groups, assessing the correlation between PMV levels and platelet aggregation, and determining the prognostic significance of PMV levels in predicting thrombotic events. By achieving these objectives, we seek to better understand the contribution of PMVs to the hypercoagulable state observed in these patients, and to explore their potential as biomarkers for identifying individuals at high risk of thrombotic complications.
2. Materials and Methods
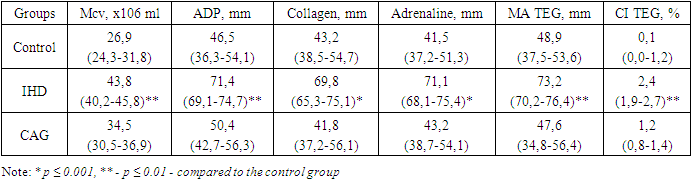
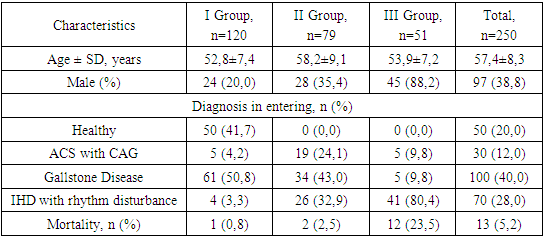
- Study DesignThis study was conducted to investigate the role of platelet microvesicles (PMVs) in the development of thrombotic complications in patients with cardiac rhythm disorders, particularly those associated with ischemic heart disease (IHD). The study comprised both a quantitative and qualitative assessment of microvesicle content across various patient groups, as well as an evaluation of the prognostic significance of PMV levels in predicting thrombotic complications.Patient SelectionA total of 250 patients were enrolled in the study, which included three groups based on PMV levels:• Group I: Patients with low PMV levels (<25 million/mL; n=120).• Group II: Patients with intermediate PMV levels (25-35 million/mL; n=79).• Group III: Patients with high PMV levels (>35 million/mL; n=51).Patients were selected based on the presence of cardiac rhythm disorders, with additional subgroups for ischemic myocardial damage, acute coronary syndrome (ACS) with coronary angiography (CAG), and healthy controls. All patients provided informed consent before participation.Sample Collection and PreparationPeripheral blood samples were collected from patients at various time points: preoperatively, immediately post-CAG, and on the 3rd and 7th days following surgical intervention. Blood samples were processed to isolate plasma, from which PMVs were subsequently extracted.Quantification and Characterization of MicrovesiclesMicrovesicles were quantified and characterized using flow cytometry, a technique that allows for the identification and analysis of particles based on size, granularity, and fluorescence. Microvesicles were labeled with fluorochrome-conjugated antibodies against specific markers, including:• Phosphatidylserine (PS): Detected using annexin V binding assays.• Platelet markers (CD41 and CD61): Used to identify platelet-derived microvesicles.• Leukocyte marker (CD45): Used to identify leukocyte-derived microvesicles.• Erythrocyte marker (CD235): Used to identify erythrocyte-derived microvesicles.• Tissue factor (TF): Used to assess the procoagulant potential of the microvesicles.Fluorescence intensity and the percentage of microvesicles positive for each marker were recorded.Platelet Aggregation AssaysPlatelet aggregation was assessed using a Multiplate analyzer, measuring the response to various agonists, including ADP, collagen, and adrenaline. The extent of platelet aggregation was correlated with PMV levels to evaluate their potential role in hypercoagulability.Prognostic AnalysisThe prognostic significance of PMV levels was evaluated by assessing their predictive value for thrombotic events. ROC curve analysis was performed to determine the sensitivity, specificity, and predictive values (positive and negative) of PMV levels in predicting thrombotic complications.Statistical AnalysisData were analyzed using SPSS software. Correlations between PMV levels and clinical outcomes, including thrombotic events and mortality, were assessed using Pearson’s correlation coefficient. ROC curve analysis was employed to determine the diagnostic accuracy of PMV levels. Statistical significance was defined as p < 0.05.
3. Results and Discussions
- In the initial phase of the study, a quantitative and qualitative assessment of microvesicle content was conducted across the groups. The hemostatic parameters of the patients under investigation are presented in Table 1.
|
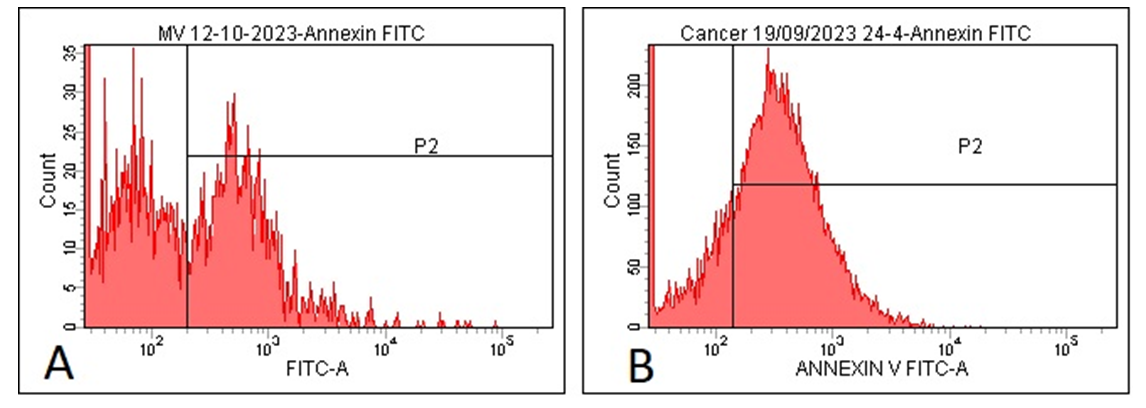 | Figure 1. Expression of Phosphatidylserine (PS) on Microvesicles in the Control Group and Patients with Ischemic Heart Disease and Cardiac |
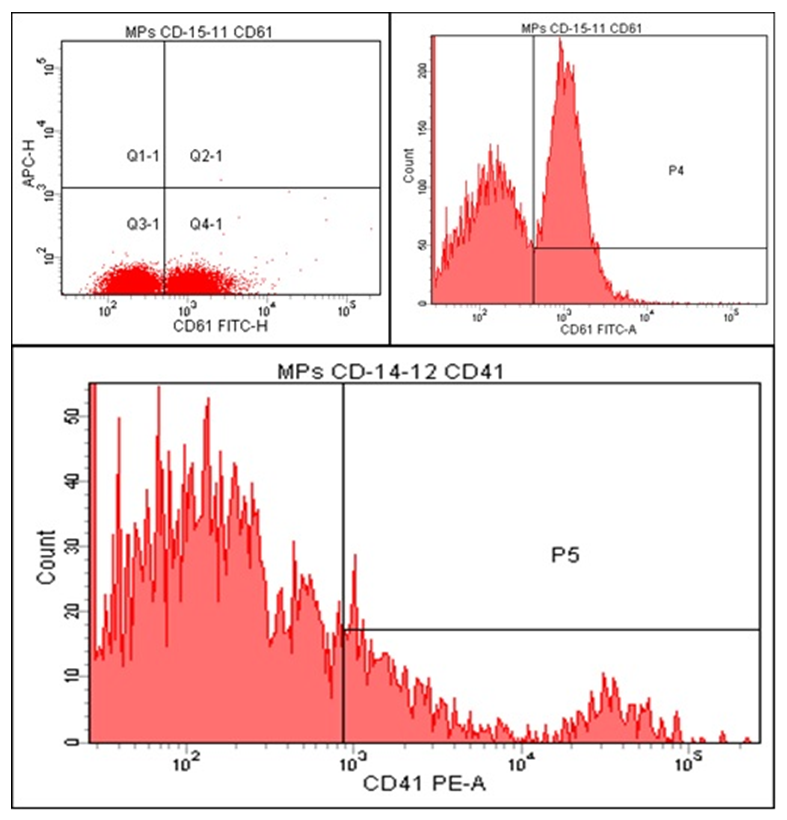 | Figure 2. Histograms of CD41 and CD61 Markers on Microvesicles in Patients with Ischemic Heart Disease and Cardiac Rhythm Disorders |
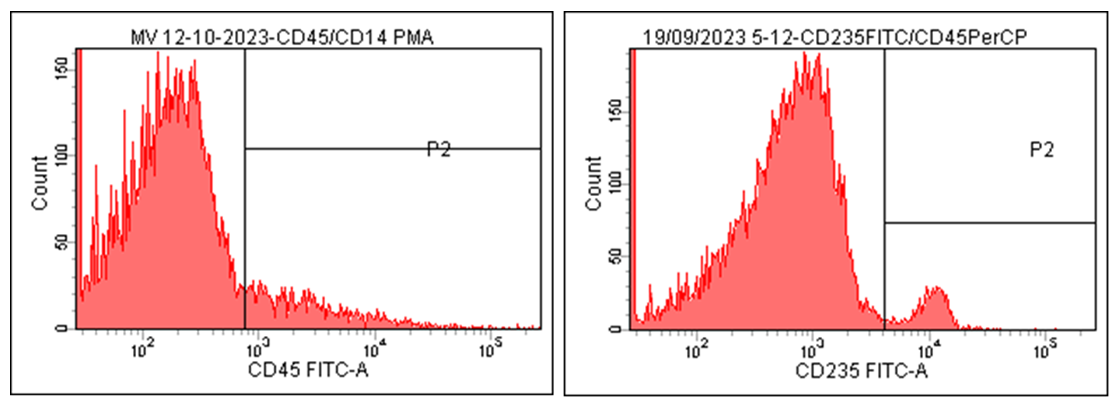 | Figure 3. Histograms of CD45 and CD235 Markers on Microvesicles in Patients with Cardiac Rhythm Disorders Associated with Ischemic Myocardial Damage |
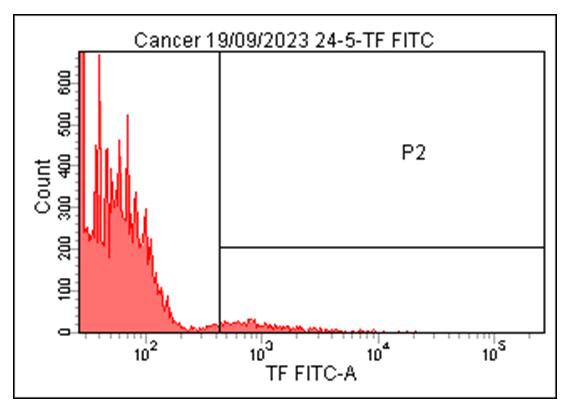 | Figure 4. Histograms of Tissue Factor (TF) Expression on Microvesicles in Patients with Cardiac Rhythm Disorders Associated with Ischemic Myocardial Damage |
|
|
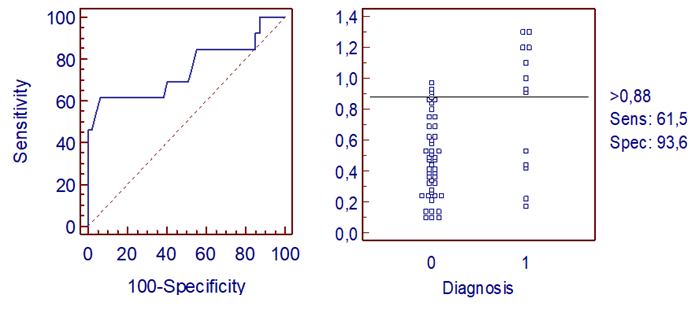 | Figure 5. Distribution of Deceased and Surviving Patients and ROC Curve of Microvesicle Content |
4. Conclusions
- The study underscores the significant role of platelet microvesicles (PMVs) in the development of thrombotic complications among patients with cardiac rhythm disorders, particularly those associated with ischemic myocardial damage. Elevated levels of PMVs, characterized by their procoagulant activity due to the presence of phosphatidylserine (PS) and tissue factor (TF), have been shown to correlate with increased risks of stroke and myocardial infarction.The findings suggest that monitoring PMV levels could serve as a valuable prognostic tool in identifying high-risk patients who may benefit from more aggressive antithrombotic therapy. However, the study also highlights the need for further research to establish standardized thresholds for PMV levels and to explore targeted therapeutic strategies that can mitigate the procoagulant effects of PMVs without compromising their physiological roles in hemostasis and immune regulation.In conclusion, this research contributes to the growing body of evidence supporting the use of PMVs as biomarkers for thrombotic risk in patients with cardiac rhythm disorders. Future studies should focus on refining the diagnostic and therapeutic applications of PMV measurement to improve patient outcomes and reduce the incidence of thrombotic complications.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML