-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(9): 2191-2194
doi:10.5923/j.ajmms.20241409.13
Received: Aug. 8, 2024; Accepted: Sep. 8, 2024; Published: Sep. 10, 2024

The Role of Periostin in the Regenerative Therapy of Periodontal Tissues in Patients with Periodontitis
K. Z. Adilov
Researcher, Tashkent State Dental Institute, Uzbekistan
Correspondence to: K. Z. Adilov, Researcher, Tashkent State Dental Institute, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Personalization of the management of patients with inflammatory periodontal diseases has been actively developed over the past decades. This is because inflammatory-destructive periodontal diseases are relevant due to their high prevalence, and also cause a decrease in the quality of life and health of the population. One of the most important conditions for the development of this area is the identification of biomarkers with high clinical informativeness. The possibilities and effectiveness of using biomarkers are determined by the choice of target, pathogenetic validity of the parameter, adequate choice of biomaterial, informativeness of testing, etc. The article presents the characteristics of one of the promising biomarkers and outlines their advantages and dependence on the severity of inflammatory and destructive processes in periodontal tissues. The rational use of biomarkers today makes it possible to identify a risk group, offer rational prevention, in some cases justify the diagnosis and assess the severity of the disease, optimize anti-inflammatory therapy, predict the response to pharmacotherapy, assess the likelihood of developing future exacerbations, and timely prescribe reconstructive surgical interventions on periodontal tissues.
Keywords: Periodontium, Inflammatory-destructive processes, Chronic generalized periodontitis, Gingival fluid, Biomarkers, Periostin
Cite this paper: K. Z. Adilov, The Role of Periostin in the Regenerative Therapy of Periodontal Tissues in Patients with Periodontitis, American Journal of Medicine and Medical Sciences, Vol. 14 No. 9, 2024, pp. 2191-2194. doi: 10.5923/j.ajmms.20241409.13.
1. Introduction
- Periostin is a secreted 90 kDa matrixcellular protein isolated from glutamate. It was first identified in the mouse osteoblastic cell line MC3T3-E1 and initially named osteoblast-specific factor 2 (OSF-2) [17]. The correct protein was renamed Periostin based on its localization in the periosteum and periodontal ligament (PDL) [19]. Periostin is highly expressed in collagen-rich fibrous connective tissues exposed to mechanical stress, such as the periosteum, LPD, tendons, heart valves, and skin. Periostin may be involved in tissue remodeling by promoting adhesion, cell differentiation, cell survival, and fibrogenesis [1].
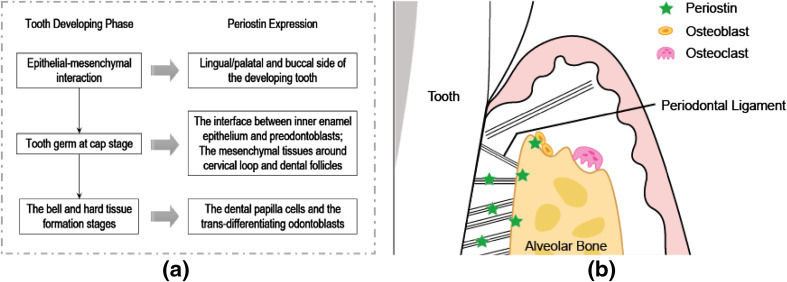 | Figure 1. Expression of periostin in periodontal tissues |
2. Material and Research Methods
- The study patients with chronic generalized periodontitis of varying severity, 54 in number, were recruited from the outpatient department of the dental clinic; the average age of the patients studied ranged from 30 to 52 years. Among them, there are 28 women and 26 men. After a clinical and radiological examination, the patients were divided into three groups as follows: a healthy group (14 people - without pathology of the dentofacial system), a group of mild, moderate, and severe chronic generalized periodontitis (18 in each group) according to the criteria of PC depth, indices OHI - S (JC Green, JR Vermillion 1964), gingival index GI (Loe, Silness 1963), PI (Rassel 1956), gingival bleeding index mSBI (HR, Muhlemann and So n 1971), orthopantomograms). The study protocol was approved by the Institutional Review Board and the Ethics Committee of the institution where the study was conducted. Informed consent was signed by all individuals after describing the need for the study. Exclusion criteria: pregnant women, nursing mothers, smokers, those who have undergone periodontal therapy in the last 6 months, taken any medications in the last 3 months, had any systemic diseases, diabetes mellitus, cancer, and diseases of the gastrointestinal tract, cardiovascular system, and thyroid gland. During the clinical examination, the following parameters were assessed: gingival bleeding index (mSBI) using the criteria given by H.R., Muhlemann, and Son, standard probing depth on each tooth from the gingival margin to the bottom of the sulcus/pocket using a William periodontal probe at 6 specific sites per tooth. The level of clinical attachment was recorded from the cementoenamel junction to the bottom of the sulcus/periodontal pocket using a William periodontal probe at all six locations as indicated for probing depth.Gingival fluid samples were obtained using microcapillary pipettes. Collection time ranged from 14 to 16 hours. The area was isolated using cotton wool/gauze. The grooved areas were carefully air-dried. A volume of 1 (microliter) was collected from each test site by an extracurricular approach using volumetric microcapillary pipettes. The collected GCF was immediately transferred to Eppendorf tubes, and venous particles (2 ml) were collected from the cubital vein, transferred to a tube coated with a coagulation activator, and centrifuged at 3000 g for 5 minutes. GCF and serum samples were stored at -70°C until analysis. Periostin levels were measured using an enzyme-linked immunosorbent assay (ELISA). It consists of 96-well plates coated with periostin-specific protectors. Standards and periostin sample solution were added to the wells.
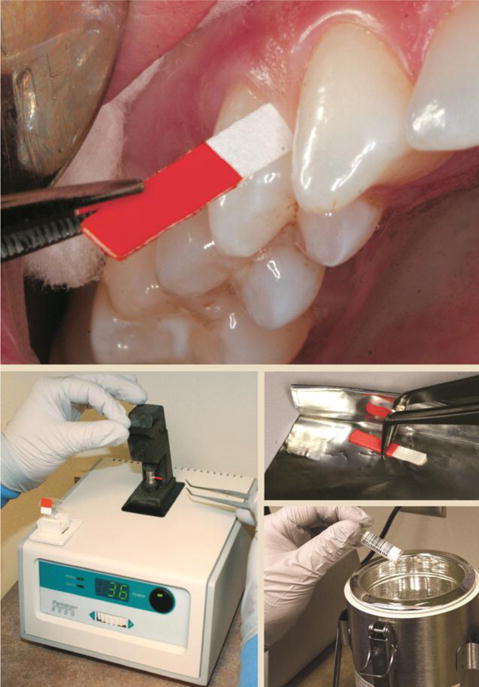 | Figure 2. Illustration of GCF sampling sequence and use of Periotron® |
3. Research Results
- Periodontal disease involves the interaction of biofilm and the immunoinflammatory response in patients, which affects the integrity of the periodontal structure and can lead to connective tissue destruction and alveolar bone resorption [12]. In this situation, indicators of gingival beard fluid are usually considered indicators of the activity of periodontal diseases in individuals [10]. Periostin gets its name from its expression in the periodontal ligament and periosteum [7]. It is an extracellular matrix protein that plays a role in connective tissue and cell connection and adhesion. Another important role of periostin is wound healing, which occurs through the interaction of type I collagen with fibronectin, thereby helping in periodontal remodeling [9]. Periostin is trapped between the cytoplasmic processes of periodontal fibroblasts, cementum areas, and also human collagen fibers [11]. It is known that periostin can regulate cell functions, promoting tissue regeneration through several signaling pathways. Periostin leads to the migration and proliferation of human fibroblasts. Moreover, periostin expression in fibroblasts promotes human mesenchymal stem cell transfer through the αvβ3-integrin/FAK/PI3K/Akt pathway in vitro [8]. In addition, periostin regulates angiogenesis through upregulation of vascular endothelial growth factor (VEGF) and MMP-2, which can be expressed through activation of the αvβ3 integrin/extracellular kinase signaling pathway in human PDL cells [4]. Consequently, a decrease in the amount of periostin directly reduces the restoration and formation of periodontal tissue. Therefore, periostin can be used as a marker of periodontal regeneration, and periostin GCF levels have been found to decrease with the progression of the periodontal disease stage [6]. Periostin is secreted by connective tissues rich in collagen and releases periostin when subjected to mechanical action [18]. Once secreted, periostin begins to bind to molecules such as tenascin-C, collagen, and BMP-1 [13]. This property applies to the extracellular matrix and increases tissue strength [14]. Periostin also has potent mitogenic mechanisms and binds to the cell membrane through integrins [16]. Periostin shows greater specificity among proteins expressed in the periodontal ligament. Since limited studies are available on changes in periostin in periodontal disease, our study aimed to compare and evaluate periostin levels in gingival beard fluid from periodontally healthy patients and chronic periodontitis patients. When comparing the group with chronic periodontitis and healthy people, the results showed that people with chronic periodontitis had significantly lower concentrations of periostin in the gingival fluid than healthy people. Also, the level of periostin in moderate and severe forms of periodontitis was significantly lower than in mild chronic periodontitis. According to Arslan R., al. [3], periostin increases due to tissue repair and regeneration efforts during periodontal disease.
4. Conclusions
- An analysis of the study results presented in (Table 1) showed that the average value of periostin in the gingival fluid decreases in direct proportion with the progression of the disease. Thus, in the group of patients with mild CGP, the level of periostin was significantly higher than in the group with moderate severity of CGP, and in the group with moderate severity of CGP, it was also significantly higher than in the group with severe severity. Considering that the results of our study indicate that the amount of periostin in gingival fluid is reduced under inflammatory conditions, it can be concluded that periostin plays an important role in the protection of periodontal ligament cells. In our opinion, bacterial competition and a decrease in the number of fibroblasts in patients with CGP could lead to a decrease in the level of periostin in the gingival fluid. Decreased levels of periostin in the gingival fluid may compromise the stability of the periodontal ligament and aggravate the inflammatory process due to a decrease in the structural and biochemical capacity of the periodontal ligament. Periostin is required for patients and diseased periodontal ligaments during occlusal loading in mice [16]. Periostin null mice exhibit dwarfism, incisor enamel defects, and an early periodontal disease phenotype [15]. Based on the preliminary understanding of the effect of periostin on the healing process of periodontal tissues [12], studies have also reported that increased levels of periostin in gingival fluid were observed after periodontal surgery, and this increase was associated with a decrease in chronic inflammatory stimuli after the surgical procedure. Also, given that gingival sampling is a minimally invasive method, by sampling and measuring the concentration of periostin in gingival fluid, useful information can be obtained for the early detection of periodontal diseases to provide timely and effective treatment. The study recognizes that the level of periostin in gingival fluid can be considered a potential biomarker in terms of periodontal disease activity.
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML