-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(9): 2183-2190
doi:10.5923/j.ajmms.20241409.12
Received: Aug. 5, 2024; Accepted: Sep. 7, 2024; Published: Sep. 10, 2024

Dynamics of Cellular Immunity Indices in Kidney Damage in Patients Carried SARS-CoV2
Xursandov Ilyos Axmedovich1, Кhamdamov Bakhtiyor Zarifovich2, Khamdamov Ilkhomjon Bakhtiyorovich2, Кhamdamova Muhayyoxon To`xtasinovna2
1Multidisciplinary Medical Center “Sultan Hospital", Termez, Uzbekistan
2Bukhara State Medical Institute, Bukhara, Uzbekistan
Correspondence to: Кhamdamov Bakhtiyor Zarifovich, Bukhara State Medical Institute, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Relevance. The SARS-CoV-2 coronavirus has evolved into a life-threatening pandemic disease, Covid-19. Acute respiratory distress syndrome (ARDS) and diffuse alveolar involvement are known to be the main manifestations of this disease. Purpose of the study: To study the indices of cellular immunity in renal injury in SARS-CoV2 patients. Material and methods of research: The paper presents data on the comprehensive examination and treatment of 62 patients with kidney injury who underwent SARS-CoV-2. The distribution of patients was based on a prospective targeted open randomized study. The period of research and collection of clinical material started in the second quarter of 2020 and ended in December 2023. Conclusion. Thus, acute kidney damage in patients after SARS-CoV2 occurs against the background of a low number and activity of CD4+ cells and a high value of CD8+, CD19+ cells, which ultimately leads to impaired regeneration of individual parts of damaged nephrons, stimulating the growth and accumulation of circulating immune complexes and changing their antigenic structure.
Keywords: Cellular immunity, Kidney damage SARS-COV2, Regeneration
Cite this paper: Xursandov Ilyos Axmedovich, Кhamdamov Bakhtiyor Zarifovich, Khamdamov Ilkhomjon Bakhtiyorovich, Кhamdamova Muhayyoxon To`xtasinovna, Dynamics of Cellular Immunity Indices in Kidney Damage in Patients Carried SARS-CoV2, American Journal of Medicine and Medical Sciences, Vol. 14 No. 9, 2024, pp. 2183-2190. doi: 10.5923/j.ajmms.20241409.12.
Article Outline
1. Relevance
- The SARS-CoV-2 coronavirus has evolved into a life-threatening pandemic disease, Covid-19. Acute respiratory distress syndrome (ARDS) and diffuse alveolar involvement are known to be the main manifestations of this disease [1,3,5,7,9,11,13,15]. Although the respiratory system is the primary target of SARS-CoV-2, other organs in the body can be affected by the virus via the circulatory system. Initially, information on kidney involvement (KI) was sparse. Publications regarding PP in SARS-CoV-2 early in the pandemic were not systematic and were characterized by isolated clinical cases ranging from mild proteinuria to progressive acute renal failure (ARF) [2,4,6,8,10,12,14,16,18,20]. During SARS-CoV-2 infection, decreased CD4+ and CD8+ cell counts and increased cytokine levels cause inflammation [17,19]. Regarding the specificity of SARS-CoV-2 virus, different T cell subsets are activated [18]. The overproduction of cytokines that leads to ARDS is associated with the outcome and severity of SARS-CoV-2. The enhanced inflammatory response induced by SARS-CoV-2 plays a key role in the severity of infection, development of PP and death [3].
2. Purpose of the Study
- To study the indices of cellular immunity in renal injury in SARS-CoV2 patients.
3. Material and Methods of Research
- The paper presents data on the comprehensive examination and treatment of 62 patients with kidney injury who underwent SARS-CoV-2. The distribution of patients was based on a prospective targeted open randomized study. The period of research and collection of clinical material started in the second quarter of 2020 and ended in December 2023. At the same time for the period from April to August 2020 the clinic functioned still as a specialized covid center, with involvement of specialists of all directions according to quarantine requirements. All patients were combined into one main group. Criteria for inclusion of patients in the main group were: age of patients not younger than 20 and not older than 75 years; presence in the history of coronavirus infection disease, with severe course, with signs of kidney damage during the treatment period; persistence of signs of kidney disease (proteinuria, albuminuria, micro- or macrohematuria, decreased rate of glomerular filtration, high values of creatinine and urea in blood, etc.); presence of negative results of PCR in the clinic.); negative PCR test result for SARS-CoV-2 during hospitalization in our clinic; voluntary informed consent of the patient to participate in the clinical trial. Due to the fact that the patients of the main group came to the clinic with different duration of the anamnestic period of the disease development and the time of SARS-CoV-2 before us, there was a need to randomize the main group itself. Based on the principles of evidence-based medicine the main group of patients was subdivided by us into two subgroups:The first subgroup - 34 (54.8%) patients who were transferred from a specialized covid infectious disease clinic after achieving a negative PCR test result for SARS-CoV-2 with signs of kidney damage and the need for renal replacement therapy. The second subgroup - 28 (45.2%) patients who were transferred from other therapeutic clinics or hospitalized as in the primary treatment for the underlying disease. All of them had a history of SARS-CoV-2 and were treated as inpatients in a specialized covid infectious disease clinic. During treatment, they were diagnosed with acute kidney injury, but remission of complications was achieved by the time of hospital discharge. However, later, after 1 to 3 months, the patients came to our clinic with the need for renal replacement therapy. The chronology of hospitalization of patients with kidney injury after SARS-CoV-2 showed that patients of the first subgroup were hospitalized in our clinic predominantly (70.6%) during the peak of the first and second waves of the COVID-19 pandemic, whereas patients of the second subgroup sought specialized care predominantly after the passage of the COVID-19 pandemic waves for the period 2021-2023 (92.9%).In the comparative evaluation of clinical and immunological changes, the data of 20 healthy individuals recognized by the medical commission as absolutely healthy were used. All of them were combined into a control (reference) group.The criteria for inclusion of volunteers in the control group were: age of patients from 20 to 50 years; absence of any chronic somatic or psychiatric diseases; obligatory conclusion of the medical commission about healthy condition during the last 3 months before the study; absence of diseases from the urinary system in the anamnesis; absence of SARS-CoV-2 in the anamnesis; negative result of PCR-test for SARS-CoV-2; voluntary informed consent for participation in the clinical trial. The diagnosis of kidney damage was established according to the recommendations of the Global Foundation for the Improvement of Kidney Diseases (KDIGO) in the presence of one of three signs of pathological process manifestation in the form of: increase of serum creatinine level at 26.5 μmol/l and higher within 48 hours; increase of serum creatinine above 1.5 times compared to the baseline level during the previous 7 days; decrease of urine output less than 0.5 ml/kg/hour within 6 hours. All patients in the main group were subdivided according to the stages of kidney damage after SARS-CoV-2, which were also recommended by KDIGO. As our data showed, the third stage of renal damage prevailed among the patients of the main group (62.9%), in which the level of creatinine in the blood of patients increased 3 times from the baseline or the beginning of renal replacement therapy. In the remaining 37.1% of cases, patients had stage 1 and 2 kidney damage after SARS-CoV-2. From 17.7% of cases the first stage of kidney damage was diagnosed, when blood creatinine level increased 1.5-1.9 times from baseline, and in 19.4% of cases - 2 - 2.9 times under the same conditions. Distribution of patients by age showed predominance of patients in the age category of 51 and older (67.8%). At the same time between patients in the age category from 51 to 60 years and from 61 to 75 years the distribution of the number of patients was the same (21 patients or 33.9% each). The second place was occupied by patients aged 41-50 years (24.2%). Patients in young age were only in 8.1% of cases. It should be noted that among the patients of the first subgroup prevailed in the age category from 51 to 60 years, and in the second subgroup - from 61-75 years. Thus, renal damage was predominantly characteristic of mature and elderly patients, while acute kidney injury after SARS-CoV-2 was characteristic of younger age. Male patients predominated (67.7%), both among patients of the first and second subgroups.When performing renal replacement therapy, we used a capillary dialyzer. Special multi-stage purified water was used for dialysis according to the approved standards of the Ministry of Health of the Republic of Uzbekistan. Devices for renal replacement therapy contained a set of hydraulic pumps for mixing solutions containing electrolytes Na+, K+, Ca++, Mg++, Cl-. General clinical laboratory, biochemical, bacteriological, molecular genetic (PCR) studies were performed in a centralized clinical diagnostic laboratory. The calculation of the glomerular filtration rate (ml/hour/kg) was performed according to the formula: Club filtration rate (ml/hour/kg) = [Urine volume (ml) x urine creatinine (mg/mL)] / [time (hour) x serum creatinine (mg/mL) x patient weight (kg)].All patients underwent ultrasound examination of the kidneys in a functional diagnostics room. To determine morphostructural changes in the kidneys, all patients of the main group underwent percutaneous puncture biopsy of the kidneys in the surgical department under aseptic conditions. The biopsy material was investigated by standardized histological technique with staining under light in the conditions of the central research laboratory.Immunologic studies were performed in the Bukhara branch of the Institute of Human Immunology and Genomics. Peripheral blood served as the object of study. A 6 ml sample of venous blood was collected at one time into tubes with heparin (20 U/mL) and 5 ml into a dry tube for subsequent serum separation. The immune status and cytokine profile of the patients were assessed twice - at the initial visit of the patients to us, which usually occurred on the 1-2 day of inpatient treatment and after the treatment before discharge from the hospital, which usually occurred on average on 14-21 days of treatment. When assessing the immunologic status of the study indicators of cellular (leukocytes, neutrophils, eosinophils, monocytes, lymphocytes (CD3+), T-helpers (CD4+), T-killers (CD8+), B-lymphocytes (CD19+), phagocytic number and phagocytic index, circulating immune complexes) and humoral (immunoglobulins A, M, G and cytokines IL-1β, TNF-α, IL-8, IL-10 and IFN-γ) immunity. Quantitative determination of cytokines IL-1β, TNF-α, IL-8, IL-10 and IFN-γ was performed in blood serum using ELISA test systems of Cytokin LLC (St. Petersburg) on an automatic analyzer for enzyme-linked immunosorbent assay.Mononuclear cells were isolated on a density gradient of ficollaverographin. To identify lymphocytes and their subpopulations, immunophenotyping of mononuclear cells was performed by direct immunofluorescence using monoclonal antibodies CD3+, CD4+, CD8+, CD19+.Determination of the concentration of immunoglobulins IgM, IgG, IgA in blood serum was carried out by immunoturbometric method using an automatic biochemical analyzer. Determination of the concentration of circulating immune complexes in serum was carried out by polyethylene glycol-6000 precipitation method according to the method of Y.A. Grinevich and A.N. Alferov, 1981. Assessment of phagocytic activity included the use of leukocyte suspension, which was incubated with standard latex particles with a diameter of 1.35 μm (Immunoscreen, Moscow) for 30 min, swabbed, stained with Azur-eosin and counted the number of neutrophils that captured latex particles. The percentage of phagocytizing neutrophils was denoted as phagocytic index. The phagocytic number was determined, which corresponded to the number of particles taken up on average by one phagocytic neutrophil.In order to assess the prognostic significance of the studied parameters of immunologic disorders in patients with kidney damage after SARS-CoV-2 we used the method of calculating the dependence between multivariate variables and the model created by the statistical model for predicting the disorder. This analysis was performed by constructing linear regression analysis. Factor analysis was used to examine the mutual relationship of the various traits studied, which determined the variance of specific factors in the form of a variance cloud. Statistically significant linear combinations of a number of factors allowed us to reduce the dimensionality of the trait graphical cloud that can be analyzed. The linear plotting of the data allowed us to compare specific factors against each other. This in turn was the key to examining the actions on the final outcome of the factors analyzed in each variance data cloud. Using this method of statistical analysis allowed us to quantify the observed signs of change. The studied factors and their constituent immunologic data were presented in quantitative form; the observed traits influenced by the studied factors were the outcome traits.
4. Results and Their Discussion
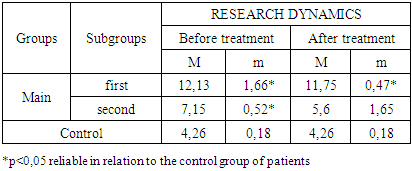
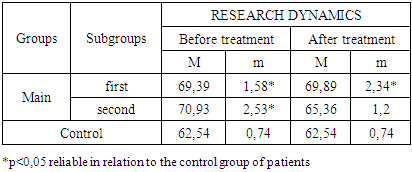
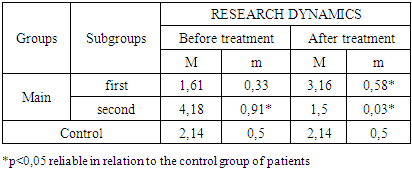
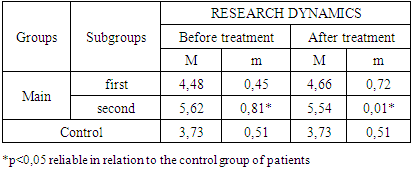
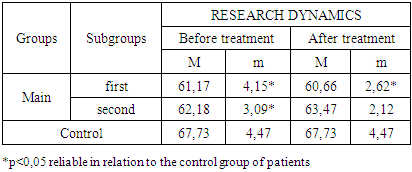
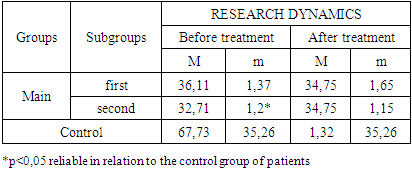
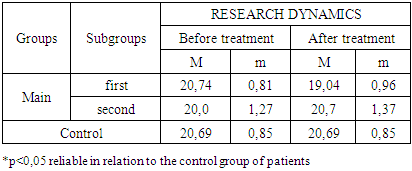
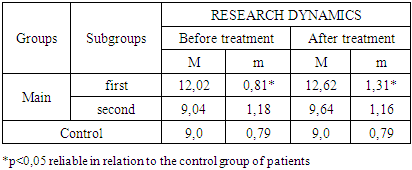
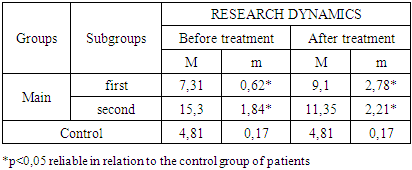
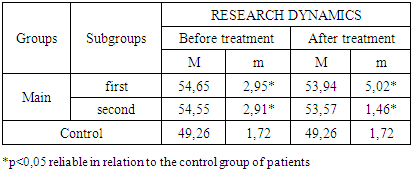
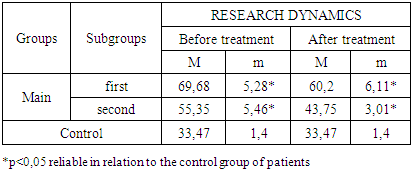
- The study of the state of cellular immunity in patients with kidney damage after SARS-CoV2 was mainly aimed at assessing their activity against viruses. At the first stage we studied the dynamics of leukocyte changes in blood (Table 1).
|
|
|
|
|
|
|
|
|
|
|
5. Conclusions
- Thus, acute kidney damage in patients after SARS-CoV2 occurs against the background of a low number and activity of CD4+ cells and a high value of CD8+, CD19+ cells, which ultimately leads to impaired regeneration of individual parts of damaged nephrons, stimulating the growth and accumulation of circulating immune complexes and changing their antigenic structure. High values of phagocytic number and index may indicate the continued destruction of the basement membranes of nephrons, which ultimately leads to a decrease in the functional volume of the kidneys with the development of chronic renal failure.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML