-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(9): 2151-2156
doi:10.5923/j.ajmms.20241409.05
Received: Aug. 17, 2024; Accepted: Sep. 2, 2024; Published: Sep. 6, 2024

Evaluation of the Role of Genetic Polymorphism of the CYP17A1 Gene (rs 743572) in the Etiopathogenesis of PCOS in the Uzbek Population
Sadirova S. S.1, Irgasheva S. U.1, Boboev K. T.2
1Republican Specialized Scientific and Practical Medical Center for "Maternal and Child Health" State Institution, Uzbekistan
2State Institution "Republican Specialized Hematology Scientific and Practical Medical Center", Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Polycystic ovary syndrome is a widespread, multifactorial and complex endocrine disease that is one of the main causes of female infertility. In recent years, there has been a tendency to increase the frequency of this pathology in the framework of disorders of the menstrual cycle and reproductive functions. Purpose of the study: to study the genetic relationship of polymorphism of the CYP17A1 gene (rs 743572) in the Uzbek population of reproductive age with polycystic ovary syndrome. Materials and methods. Association analysis of CYP17A1 gene polymorphism was carried out using a case-control model (case-control, comparison of two samples). The study examined genotyping of the polymorphic A/G locus rs 743572 of the CYP17A1 gene, associated with disorders of androgen synthesis, in 155 women aged 19-35 years with various PCOS phenotypes and healthy women with normal menstrual and generative functions. Results and its discussion. It was found that among relatively healthy donors, the percentage of A/A genotype carriers was significantly higher than in PCOS patients (55% and 39%, respectively; X2=3.9; p=0.05), and this genotype was associated with a low risk of developing PCOS (protective effect) OR = 0.4; CI95% 0.28–0.99. In addition, different frequencies of the heterozygous A/G genotype were found in the control and patient groups (36% and 45%, respectively, χ2< 3.85; p>0.05), and the probability coefficient was -1.5. (95% CI: 0.76–2.77). Different frequencies of the negative G/G genotype were also observed in the main and control groups. (16.3% and 9.3% (χ2< 3.85; p> 0.05). The odds ratio for developing PCOS in carriers of this genotype is OR = 1.9, 95% CI: 0.72-4.96. The relative risk of the disease was RR = 1.7: 95% CI 0.85-3.55. Conclusion: A/G Polymorphism of the CYP 17A1 gene is associated with the pathogenesis of PCOS. The homozygous mutant G/G genotype plays an important role in the etiopathogenesis of the disease. This genotype is considered a risk factor for the development of PCOS in the population of Uzbekistan. Carriage of the genotypic variant of the mutant genotype G/G of the CYP 17A1 gene leads to an increase in the risk of developing PCOS by more than 1.9 times.
Keywords: Polycystic ovary syndrome, CYP17A gene, Hyperandrogenism, Polymorphism, Genetic predisposition
Cite this paper: Sadirova S. S., Irgasheva S. U., Boboev K. T., Evaluation of the Role of Genetic Polymorphism of the CYP17A1 Gene (rs 743572) in the Etiopathogenesis of PCOS in the Uzbek Population, American Journal of Medicine and Medical Sciences, Vol. 14 No. 9, 2024, pp. 2151-2156. doi: 10.5923/j.ajmms.20241409.05.
1. Introduction
- Ovarian polycystic syndrome (PCOS, Stein-Leventhal syndrome) is one of the most urgent problems of gynecological endocrinology and causes female infertility. The incidence of this disease is 6-20% among the general population, 5-10% among women of reproductive age. [4] In recent years, there is a tendency to increase the frequency of this pathology as part of disorders of the menstrual cycle and reproductive functions. Hirsutism, obesity in the abdominal area, infertility and menstrual cycle disorders that develop with PCOS lead to loss of feminine attractiveness, defeminization, disturbances in the patient's emotional state, and a decrease in the quality of life. [6] Therefore, in practice, prevention of the development of PCOS in healthy teenage girls and women of reproductive age, strategies for early diagnosis and determination of genetic predisposition, restoration of menstrual cycle and reproductive functions, correction of metabolic diseases and differential restoration of rational health care of patients in the risk group of primary and secondary prevention is the basis for developing an individual program. Despite the fact that several different theories of the origin of the disease have been proposed, the pathogenesis has not yet been fully studied. In the etiology of the disease, genetic factors account for an average of 79%, and the remaining 21% are environmental, lifestyle and personal medical history. The genetic theory of the development of PCOS is relevant, modern and actively studied in the development of the disease. [1] The search for PCOS-related polymorphisms is of great interest to the scientific community. However, despite significant advances in the field of genetics and molecular biology, the emergence of new modern methods for detecting various mutations in genes, the question of the influence of genetic factors on the risk and characteristics of the development of PCOS remains open. [3] The main genes associated with the development of clinical manifestations of PCOS are represented by two groups.The first group includes genes that control the metabolic processes of carbohydrate metabolism and, accordingly, the state of hyperinsulinemia and insulin resistance.The second group includes genes responsible for the synthesis of steroid hormones and the sensitivity of individual tissues to androgens.Changes in the structure of one or more of these genes can lead to the development of certain clinical signs or symptom complexes characteristic of polycystic ovary syndrome. [2]Information about the genetic predisposition to PCOS allows the doctor to determine the cause-and-effect relationship in the appearance of various clinical manifestations of PCOS, which in turn is important in choosing treatment methods, approaching each patient individually. [5]Hyperandrogenemia has long been the reason for the assumption that there is a specific mutation in the genes encoding the enzymes of steroidogenesis. Modern studies have shown that the activity of enzyme systems, especially cytochrome P450 aromatase, is variable, which confirms the etiopathogenetic heterogeneity of the disease. Research conducted in recent years shows the identification of candidate genes that are the most common polymorphic characters and are involved in steroidogenesis processes. [A.Yu. Beglova, S.I. Elgina., N.V. Artymuk, L.A. Gordeeva 2019 Noskova I.N., Artymuk N.V., Gulyaeva L.F. 2019 Koutsotanaz Ch. Agiannitopoulos K. Georgoutsou M. Bampali K. 2017. Park J. Lee E. Ramakrishna S. Cha D. Baek K. 2018]. At the same time, the nature of changes in ovarian aromatase activity and the relationship between genetic factors affecting the formation of a certain phenotype of the disease has not been sufficiently studied, which indicates the need for research. [7] CYP11A, CYP17, CYP19, CYP21, BetaHSD polymorphisms, which play a role in steroidogenesis, lead to phenotypic expression of PCOS. In addition, androgen receptor gene AR mediates androgen levels and SGBG regulates serum free androgen levels, so all of these genes may be involved in the etiopathogenesis of PCOS. [8]The CYP 17 gene is located on chromosome 10q24-q25 and encodes the cytochrome P450 17a-hydroxylase 17, 20-lyase enzyme present in the endoplasmic reticulum. This enzyme plays a key role in the biosynthesis of steroid hormones due to its hydroxylase and lyase activity. It converts pregnenolone and progesterone into 17-hydroxypregnenolone and 17-hydroxyprogesterone, respectively, through 17α-hydroxylase and 17-20-lyase activity. In the next step, it converts these steroids into dehydroepiandrosterone and 4-androstenedione. [9,16] Many researchers have concluded that dysregulation of the P450 CYP 17 enzyme is one of the causes of ovarian hyperandrogenemia in PCOS. [Echiburú B, Pérez-Bravo F, Maliqueo M, Sánchez F, Crisosto N, Sir-Petermann. 2008. Kaur R, Kaur T, Kaur A. 2018.]In order to test the association of rs 743572 CYP17A1 gene polymorphism with steroidogenesis in the population of Uzbekistan and the hypothesis about the role of this polymorphism in the etiopathogenesis of PCOS, in patients with different phenotypes of PCOS and in healthy women with intact menstruation and generative functions we conducted an association study.The purpose of the studyGenetic association study of rs 743572 CYP17A1 gene polymorphism in Uzbek population of reproductive age with polycystic ovary syndrome.
2. Materials and Methods
- Study Design and PopulationMolecular - genetic studies were conducted in the Department of Molecular Medicine and Cell Technologies of the Republican Specialized Hematology Scientific-Applied Medical Center. Association analysis of CYP17A1 gene polymorphism was performed using a case-control model (case-control, two-sample comparison). The study was conducted with the consent of the women who applied for advice to the Polyclinic of the Republican Specialized Scientific and Practical Medical Center of Maternal and Child Health. 155 women aged 19-35 participated in the study. The "case" sample was formed from 80 patients with various PCOS phenotypes who were examined and monitored by obstetrician-gynecologist, gynecologist-endocrinologist specialists at the Polyclinic of the Republican Specialized Mother and Child Health Scientific and Practical Medical Center. The control group consisted of 75 healthy women without diseases of the menstrual cycle and reproductive system, hyperandrogenism and obesity. The diagnosis of PCOS was made according to the Rotterdam consensus criteria for PCOS (2003). The study included genotyping the A/G polymorphic locus of the rs 743572 CYP17A1 gene, which is associated with androgen synthesis disorders.Inclusion and Exclusion CriteriaInclusion criteria for the main group: women diagnosed with PCOS, reproductive age 19-35, informed for participation in the study. Exclusion criteria from the main group: women under 19 years and over 35 years; did not agree to participate in the study; women taking hormonal therapy, combined oral contraceptives. Pregnant women and women with competing diseases (congenital dysfunction of the adrenal cortex, hyperprolactinemia, hypothyroidism, idiopathic hirsutism, hypercorticism, etc.). Criteria for inclusion in the comparison group: healthy women of reproductive age without infertility, severe somatic diseases or somatic pathology during the period of compensation, without PCOS. Exclusion criteria from the comparison group: women under 19 years and over 35 years; women of reproductive age with severe somatic pathology in the stage of decompensation, infertility; refused to participate in the study; women taking hormonal therapy, combined oral contraceptives.Genetic AnalysisDNA samples from peripheral blood of healthy women and women with different PCOS phenotypes were used as material for the study. Genetic studies and analysis of the obtained data were carried out in accordance with the principles of GRIPS in order to increase the transparency and quality of risk prediction.A modified phenol-chloroform extraction method and the "RNA/DNA sorb" kit from InterLabService LLC (Russia) were used to extract DNA from peripheral blood lymphocytes. Standard vacuum tubes, Vacutainer Becton Dickinson International (USA) with EDTA were used for biomaterial collection. Isolation of lymphocyte nuclei and subsequent DNA was performed according to the method proposed by Sambrook J. (1989) with some modifications. The concentration and purity of the extracted DNA was measured on a NanoDrop 2000 spectrophotometer (USA) at A260/280 nm wavelength. The amplification reaction was carried out in the GeneAmp PCR-system 2720 thermal cycler (Applied Biosystems, USA) and CG1-96 (Corbett Research, QUAGEN Germany) using the polymerase chain reaction of DNA synthesis. NPF Litex LLC (Moscow) and InterLabServis LLC (Moscow) test systems were used for molecular genetic analysis of CYP17A1 (rs743572). Under the following conditions: denaturation (94°C - 4 min.), 94°C - 10 sec, 60°C - 20 sec, 72°C - 20 sec (30-35 cycles), final synthesis (72°C - 7 min). After completion of PSR, the specificity of amplification and the amount of amplification were checked by electrophoresis.Statistical AnalysisFrequency analysis methods: ROC, AUC, OR and HWE analyzes were used in the study of genetic material. Statistical processing of genetic data was performed using Epi Info 7.2.2.2, a universal statistical program for scientific research in the field of epidemiology. The allele frequencies of the studied genes were calculated according to the following formula:p= (2np+npq) /2N, where N is the sample size; np – number of homozygotes for p allele; npq is the number of heterozygotes.The χ2 criterion was used to compare the frequencies of alleles and genotypes in patients and control groups. The degree of associations was estimated using the odds ratio (OR) and its 95% confidence interval (95% CI).
3. Results and Their Discussion
- The main group consisted of 80 patients with different phenotypes of PCOS, and the control group consisted of 75 healthy women with normal menstrual cycle and reproductive function. The average age of women in the main group was 24.7±2.5 and the average age of women in the control group was 28.25±3.1. The mean body mass index was 28.3±1.45 kg/m² in the main group and 21.5±4.3 kg/m² in the control group. The main complaints of most women with PCOS were irregular menstrual cycles and lack of pregnancy. Among the PCOS patients who were examined, 14 (17.5%) patients had a preserved menstrual cycle. Oligomenorrhea was observed in 22 (27.5%) women. Primary infertility was noted in 28 (35%) patients. Secondary infertility was observed in 6 (7.5%) patients. At the next stage of the study, the influence of genetic factors on the risk of developing PCOS was studied.The results of the study of the frequency distribution of alleles and genotypes of the A/G polymorphism of the rs 743572 CYP 17A1 gene in patients with PCOS and in the control group, the results of the expected and observed frequencies were consistent with those expected under the conditions of Hardy-Weinberg equilibrium (HWE). The frequency of A and G alleles was 0.61 and 0.39 in the patient group and 0.73 and 0.27 in the control group, respectively.In the total group of patients with different PCOS phenotypes, the observed and expected frequencies of A / A, A / G and G / G genotypes were 0.39 / 0.38, 0.45 / 0.47 and 0.16 / 0, 15 respectively. In the comparative group, the frequency of these genotypes was 0.55/0.53, 0.36/0.4 and 0.09/0.07. It can be seen that the difference between expected and observed frequencies of genotypes in both studied groups was statistically insignificant (χ2<3.85; P>0.05).In the general group of patients, the dominant genotype of the rs 743572 CYP 17A1 gene polymorphism was the heterozygous A/G genotype, which was found in 45% of subjects. The frequency of foreign A/A and negative G/G genotypes was noted in 38.75% and 16.25% of patients. In the group of conditionally healthy donors, the dominant genotype of this locus was the homozygous A/A genotype, which was expressed with a frequency of 54.67%. The second most common heterozygous genotype was A/G, with a frequency of 36%. The frequency of the minor negative genotype G/G for this cohort was 9.33% (Figure 1).
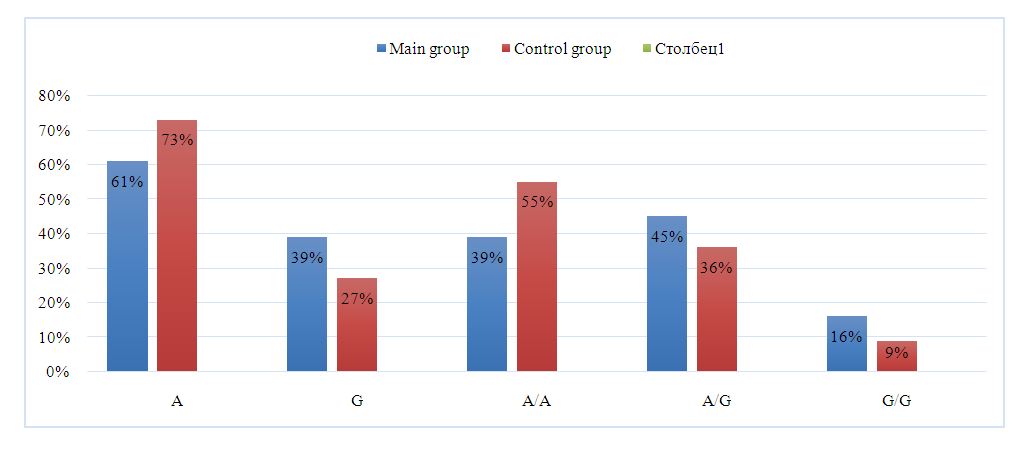 | Figure 1. Frequencies of rs 743572 CYP 17A1 gene A/G polymorphism alleles and genotypes in groups of women with PCOS and healthy women |
|
4. Conclusion
- The results of the study of the A/G polymorphism of the CYP17A1 gene showed that allele -A was statistically less observed in the patient group than in the control group. (72.7% versus 61.3%, respectively); χ2=4.5; p=0.05). Carrying the negative G allele, on the other hand, was significantly more frequent in the group of patients with PCOS than in the control group, and was 39% versus 27% (χ2 = 4.5 and p = 0.05). The presence of this allele increases the susceptibility to PCOS and causes a 1.7-fold increase in the risk of developing the pathology.Among healthy donors, it was observed that the percentage of carriage of the A/A genotype was significantly higher than in patients with PCOS (55% and 39%, respectively; χ2=3.9; p=0.05), and this genotype was associated with a lower risk of developing PCOS (protective effect). It was also known that the negative G/G genotype predisposes to the development of PCOS. Its frequency was much higher in the patient group than in the control group. (16.3% and 9.3% (χ2< 3, 85; p>0.05), but it was statistically unreliable. This genotype is considered a risk factor in the development of PCOS in the population of Uzbekistan. CYP 17A1 Carrying the G/G genotypic variant of the gene leads to a more than 1.9-fold increase in the risk of developing PCOS.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML