-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(8): 2116-2122
doi:10.5923/j.ajmms.20241408.35
Received: Aug. 9, 2024; Accepted: Aug. 26, 2024; Published: Aug. 30, 2024

The Role of Ovarian Microbiota in the Diagnosis and Treatment of Empty Follicle Syndrome in Women
Olimova K. J.1, Shukurov F. I.2
1Assistant of the Department of Obstetrics and Gynecology Termez Branch, Tashkent Medical Academy, Tashkent, Uzbekistan
2Doctor of Medical Sciences, Head of the Department of Obstetrics and Gynecology, Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Shukurov F. I., Doctor of Medical Sciences, Head of the Department of Obstetrics and Gynecology, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Aim. To evaluate the role of ovarian microbiota in the diagnosis and treatment of Empty Follicle Syndrome (EFS) and to develop methods to improve IVF outcomes. Materials and methods. The study included 110 women with a history of Empty Follicle Syndrome: Group I consisted of 40 women of early reproductive age (18-35 years), Group II included 40 women of late reproductive age (36-41 years). The control group consisted of 30 healthy women without reproductive disorders. All patients underwent clinical, laboratory, and instrumental examinations. Illumina MiSeq sequencers were used to analyze the composition of the ovarian follicle microbiota. Polymerase chain reaction (PCR) was used for the identification and quantification of microorganisms, and metagenomic sequencing was used for microbiota analysis. Results. Analysis of the ovarian follicle microbiota composition revealed significant differences between the groups. Women with EFS (Groups I and II) showed a significant decrease in microbiota diversity compared to the control group (p<0.05). In the control group, the microbiota was predominantly composed of Lactobacillus species, indicating a healthy microbiome. Group I predominantly had Lactobacillus species: Lactobacillus crispatus (45%), Lactobacillus gasseri (25%), and Lactobacillus jensenii (20%). Additionally, Bifidobacterium spp. (5%), Streptococcus spp. (3%), and Enterococcus spp. (2%) were present. Group II showed a higher concentration of pathogenic bacteria, such as Gardnerella (20%), Atopobium (15%), Prevotella (5%), Mobiluncus (10%), Megasphaera (5%), Sneathia (5%), Dialister (5%), and Mycoplasma (5%). These microorganisms are often associated with bacterial vaginosis and inflammatory conditions, which can negatively impact reproductive function and contribute to the development of EFS. Conclusion. Our study confirms the significant impact of ovarian microbiota on the success of in vitro fertilization (IVF) programs in women with Empty Follicle Syndrome. We identified pronounced differences in the follicle microbiota composition between women with EFS and healthy women. Dysbiosis of the ovarian microbiota in women with EFS negatively affects IVF success rates, as evidenced by reduced microbiota diversity and the predominance of pathogenic bacteria such as Gardnerella (20%), Atopobium (15%), Prevotella (5%), Mobiluncus (10%), Megasphaera (5%), Sneathia (5%), Dialister (5%), and Mycoplasma (5%). The use of eubiotic therapy aimed at correcting microbiota showed improved reproductive outcomes in women with EFS. Treatment with Saccharomyces boulardii CNCM I-745 lyophilizate increased IVF success rates to 75% in the early reproductive age group and to 73% in the late reproductive age group, compared to 35% and 20% respectively before treatment.
Keywords: Empty Follicle Syndrome, Ovarian microbiota, IVF, Eubiotic therapy, Saccharomyces boulardii, Reproductive health
Cite this paper: Olimova K. J., Shukurov F. I., The Role of Ovarian Microbiota in the Diagnosis and Treatment of Empty Follicle Syndrome in Women, American Journal of Medicine and Medical Sciences, Vol. 14 No. 8, 2024, pp. 2116-2122. doi: 10.5923/j.ajmms.20241408.35.
Article Outline
1. Introduction
- Empty Follicle Syndrome (EFS) is one of the most complex and understudied problems in reproductive medicine [1,2]. This condition is characterized by the absence of oocytes in the follicles after ovulation stimulation, which poses a significant barrier to the successful implementation of in vitro fertilization (IVF) programs in women [3,4]. EFS was first described in 1986, and since then, its diagnosis and treatment have been the subject of numerous studies [5,6]. Since the first description of EFS, the understanding of this condition has deepened significantly [7]. Various factors, such as genetic, hormonal, and immunological, can influence the occurrence of EFS [8,9]. In recent years, scientists have begun to study the ovarian microbiota, and initial results indicate its important role in reproductive function [8,9]. Microbiota plays a crucial role in various physiological processes of the body, including digestion, immune response, and metabolism regulation [10,11]. Research has established that the gut and vaginal microbiota significantly affect women's reproductive health. For instance, dysbiosis of the vaginal microbiota is associated with an increased risk of miscarriages, preterm births, and reproductive system infections [12,13].Recent studies show that ovarian microbiota can influence folliculogenesis and ovulation [14,15]. These studies suggest the potential of using microbiota as a biomarker for diagnosis and targeted therapy [16,17]. Given the current data, there is a need for further research to gain a deeper understanding of the role of ovarian microbiota in the pathogenesis of EFS [18,19]. However, despite significant progress, there are still no standardized approaches to the diagnosis and treatment of EFS based on microbiota analysis [20].Conducting this study will allow the development of new diagnostic criteria and treatment approaches, which ultimately can improve the effectiveness of IVF programs and reproductive outcomes in women with this syndrome.The aim of this study is to evaluate the role of ovarian microbiota in the diagnosis and treatment of Empty Follicle Syndrome and to develop methods to improve IVF outcomes.
2. Materials and Methods
- The study included 110 women with a history of Empty Follicle Syndrome (EFS): Group I consisted of 40 women of early reproductive age (18-35 years), Group II included 40 women of late reproductive age (36-41 years). The control group consisted of 30 healthy women without reproductive disorders. Inclusion criteria: women with a confirmed diagnosis of Empty Follicle Syndrome, aged 18 to 41 years, and signed informed consent to participate in the study. Exclusion criteria: acute infectious disease, severe systemic diseases, intake of antibiotics or probiotics within the last month, oncological diseases of the reproductive system. Patients were divided into groups based on age and presence of reproductive disorders. The study had a cross-sectional design. All participants underwent ultrasound examination to assess ovarian condition and provided samples for follicular microbiota analysis.The study utilized a Samsung Medison W10 ultrasound system (Korea) with a transvaginal probe for ovarian assessment. Laboratory equipment included Illumina MiSeq sequencers for analyzing the composition of follicular microbiota. The research methods included polymerase chain reaction (PCR) for identifying and quantifying microorganisms and metagenomic sequencing for microbiota analysis.The sequencing methodology involved several stages. First, follicular fluid was collected by ovarian puncture under ultrasound guidance. DNA was extracted from the follicular fluid using the QIAamp DNA Mini Kit (Qiagen) according to the manufacturer’s instructions. PCR amplification was then performed using primers specific for bacterial 16S rRNA genes. PCR products were sequenced on the Illumina MiSeq platform. Library preparation for sequencing was carried out using the Nextera XT DNA Library Preparation Kit. The Illumina MiSeq platform provided high accuracy and data coverage. The resulting sequences were analyzed using QIIME2 software. Taxon classification was conducted using the GreenGenes and SILVA algorithms. Quantitative analysis of the microbiota composition included the assessment of the relative abundance of bacteria and analysis of alpha and beta diversity. For the prevention and treatment of EFS, a preparation containing Saccharomyces boulardii CNCM I-745 lyophilizate was used. The preparation was administered orally, one sachet twice a day for 15 days.The following statistical analysis methods were used to process the data: ANOVA for comparing mean values between groups; Pearson's correlation coefficient to assess the relationship between microbiota composition and clinical parameters; regression analysis to determine the influence of various factors on treatment outcomes; multivariate data analysis, including cluster analysis and principal component analysis, to identify sample groupings and patterns in the data.
3. Research Results
- In Group I, consisting of 40 women of early reproductive age (18-35 years), the average age was 29.3±3.2 years. In Group II, including 40 women of late reproductive age (36-41 years), the average age was 38.7±2.4 years. The control group included 30 healthy women without reproductive disorders, with an average age of 30.1±3.5 years.Analysis of the ovarian follicle microbiota composition revealed significant differences between the groups. Women with EFS in Groups I and II showed a significant decrease in microbiota diversity compared to the control group (p<0.05). In the control group, the microbiota was predominantly composed of Lactobacillus species, indicating a healthy microbiome.In Group I, the predominant bacteria were Lactobacillus species: Lactobacillus crispatus (45%), Lactobacillus gasseri (25%), and Lactobacillus jensenii (20%). Additionally, Bifidobacterium spp. (5%), Streptococcus spp. (3%), and Enterococcus spp. (2%) were present.In Group II, there was an increased concentration of pathogenic bacteria, such as Gardnerella (20%), Atopobium (15%), Prevotella (5%), Mobiluncus (10%), Megasphaera (5%), Sneathia (5%), Dialister (5%), and Mycoplasma (5%). These microorganisms are often associated with bacterial vaginosis and inflammatory conditions, which can negatively impact reproductive function and contribute to the development of EFS.In the control group, Lactobacillus spp. predominated: Lactobacillus crispatus (60%), Lactobacillus gasseri (25%), and Lactobacillus jensenii (10%). The absence of pathogenic bacteria and the dominance of Lactobacillus spp. indicate a healthy microbiota condition in women in this group. The results of the ovarian follicle microbiota analysis are presented in Figure 1.
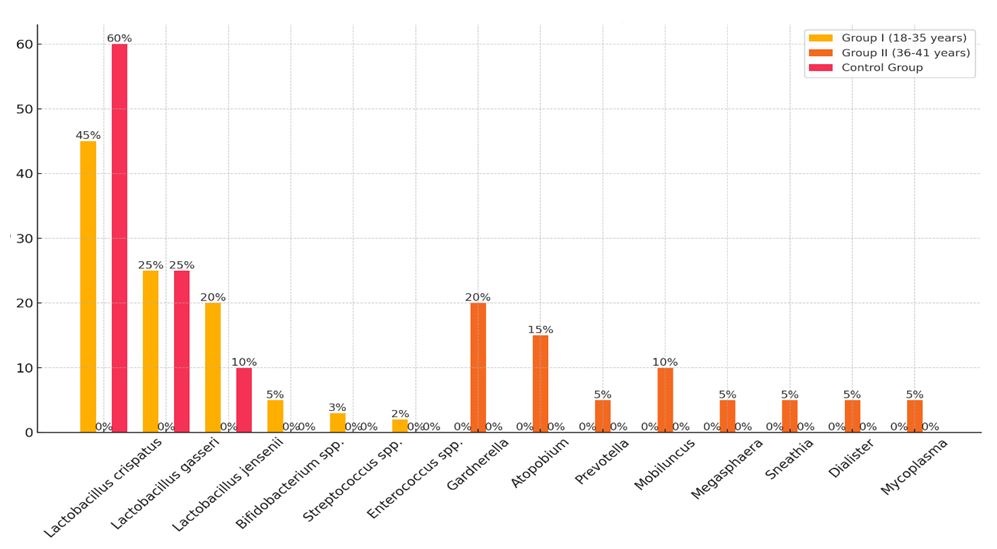 | Figure 1. Composition of Ovarian Follicle Microbiota in Women with EFS and in the Control Group |
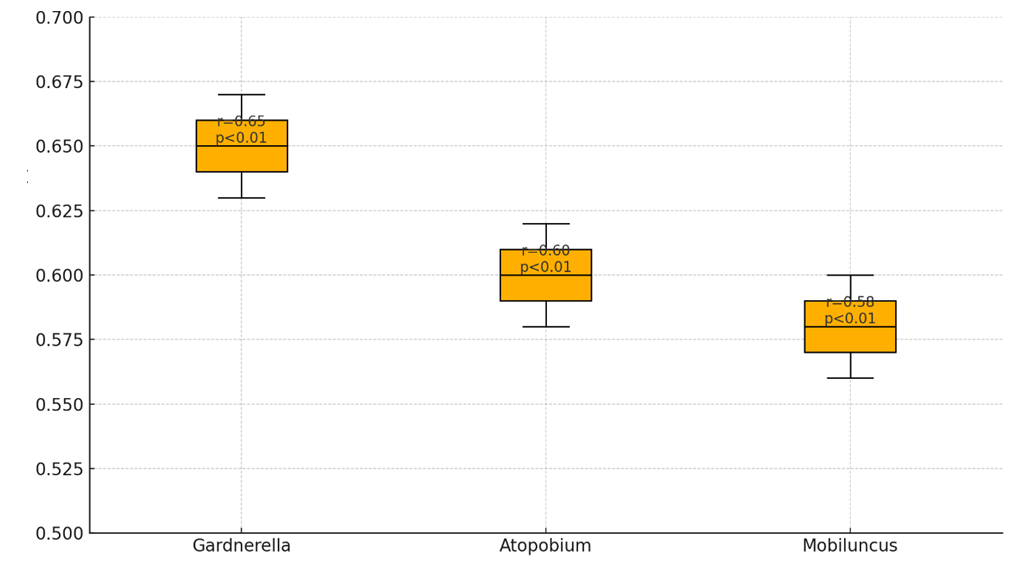 | Figure 2. Correlation of Bacterial Levels with the Development of EFS |
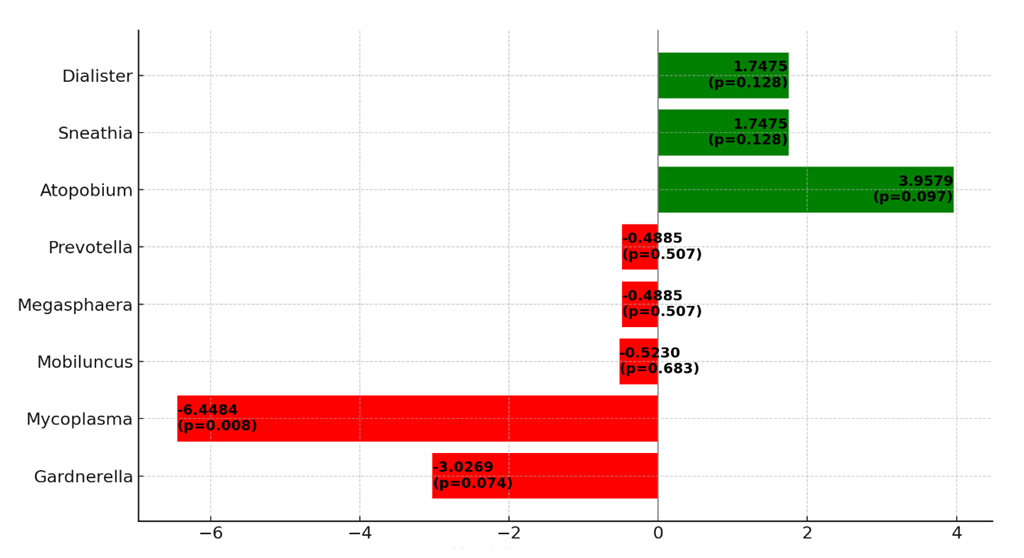 | Figure 3. Impact of Different Bacteria on the Success of IVF Programs |
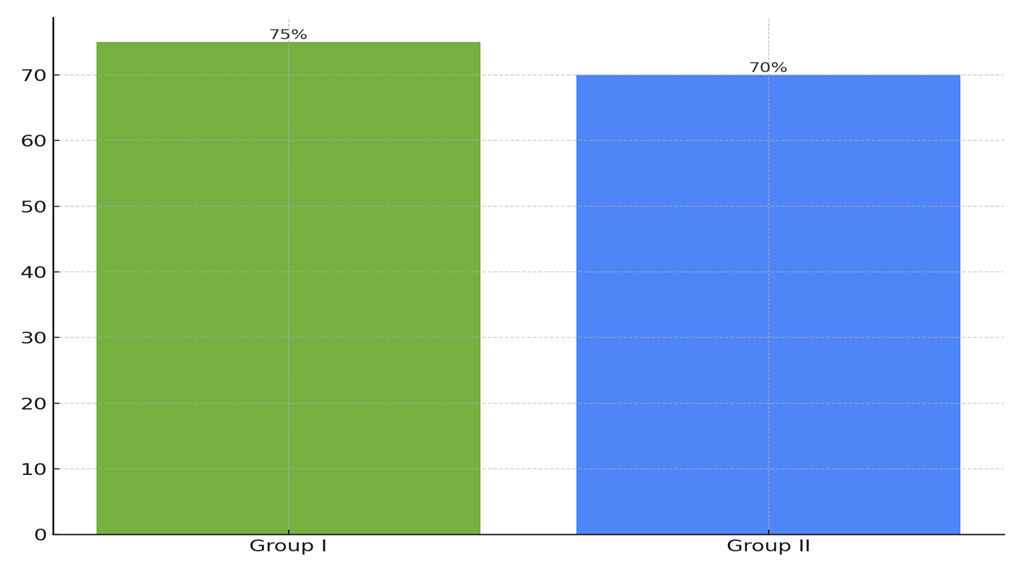 | Figure 4. IVF Outcomes Before and After Probiotic Therapy |
4. Discussion
- The obtained results demonstrate the significant influence of ovarian microbiota on the success of IVF programs. The primary new aspect of our study is the clear link between microbiota dysbiosis and reduced IVF success rates. These data support the hypothesis that ovarian microbiota plays a key role in reproductive function and can serve as an important marker for diagnosing and treating reproductive disorders. One of the key findings is that women with normal microbiota (Group I) have an IVF success rate of 35%, while women with pronounced dysbiosis (Group II) have a success rate of 20% (p<0.05). This finding underscores the importance of assessing ovarian microbiota when planning IVF programs. Our study also showed that correcting microbiota with probiotic therapy significantly improves reproductive outcomes. In Group I, IVF success increased to 75% after treatment, and in Group II, it increased to 70% (p<0.05). The practical significance of the obtained results lies in the potential use of ovarian microbiota as a diagnostic marker for assessing IVF failure risk. Implementing probiotic therapy can become an effective method for improving reproductive outcomes in women with Empty Follicle Syndrome. Clinical recommendations may include regular monitoring of ovarian microbiota in patients planning IVF and using probiotics to correct dysbiosis. In conclusion, our study highlights the importance of ovarian microbiota in reproductive medicine and opens new perspectives for diagnosing and treating reproductive disorders. The use of microbiological markers and probiotic therapy can significantly improve IVF program outcomes and increase the chances of successful pregnancy in women with Empty Follicle Syndrome.
5. Conclusions
- Our study confirms the significant influence of ovarian microbiota on the success of IVF programs in women with Empty Follicle Syndrome. We identified marked differences in the composition of follicular microbiota in women with EFS and healthy women. Ovarian microbiota dysbiosis in women with EFS negatively affects IVF success rates, as evidenced by decreased microbiota diversity and the predominance of pathogenic bacteria such as Gardnerella (20%), Atopobium (15%), Prevotella (5%), Mobiluncus (10%), Megasphaera (5%), Sneathia (5%), Dialister (5%), and Mycoplasma (5%). The use of probiotic therapy aimed at correcting microbiota showed improved reproductive outcomes in women with EFS. The administration of Saccharomyces boulardii CNCM I-745 lyophilizate increased IVF success rates to 75% in the early reproductive age group and 70% in the late reproductive age group, compared to 35% and 20%, respectively, before treatment (p<0.05). The obtained results support the role of ovarian microbiota in the pathogenesis of EFS and open new possibilities for using microbiota as a diagnostic and therapeutic marker. Implementing probiotic therapy can become an effective method for correcting dysbiosis and increasing IVF success rates.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML