Komilov Nematulla1, Nematov Aminjon2
1Center for the Development of Professional Qualifications of Medical Workers of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
2Department of Epidemiology, Center for the Development of Professional Qualifications of Medical Workers of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Komilov Nematulla, Center for the Development of Professional Qualifications of Medical Workers of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Activation of natural CCHF foci with epidemic manifestations necessitates an in-depth study of a number of issues regarding the epizootology and epidemiology of this disease, which is of great importance for improving and differential approach to planning and carrying out preventive and anti-epidemic measures, which determined the purpose of our study. Background: The purpose of the work is to study the biostructure of natural CCHF foci, the level of natural infection of rodents and ticks with the CCHF virus in order to determine the risk of population infection with the virus and predict changes in the activity of natural foci. Methods: Virological methods (infection of VERO E-6 cell culture, newborn white outbred mice - NBM), molecular genetic (polymerase chain reaction - PCR), serological (enzyme-linked immunosorbent assay - ELISA, agar gel immunodiffusion test - AGID, indirect hemagglutination - IHA) were used). When diagnosing IHA, we used the erythrocyte immunoglobulin diagnosticum (EIGD) for the CCHF virus. Results: The paper presents the results of laboratory studies of about 35,000 specimens of ticks, combined into 2,961 pools and 6,244 samples of rodent blood serum for infection with the Crimean Congo hemorrhagic fever virus (CCHF). Differences in the biostructure of natural foci, the intensity of the epizootic process and the polyvector nature of the circulation of the CCHF virus are shown depending on the regions, differing in climatic, geographical and social conditions. In the Republic as a whole, the most infected species of wild rodents are the great gerbil (Rhombomus opimus), midday jird (Meriones meridianus) and Libyan jird (Meriones libycus), ticks - H. anatolicum, H. detritum, H. asiaticum. Conclusions: A high level of CCHF virus infection in common tick species may indicate a high degree of danger from contact with them for farm animals and people. These indicators are prerequisites for the maturation of the epizootic process with the subsequent transition to epidemic manifestation.
Keywords:
Crimean Congo hemorrhagic fever, Serodiagnosis, Rodents, Ticks, Natural focus, Epizootic process
Cite this paper: Komilov Nematulla, Nematov Aminjon, Results of Epizootological Studies of Natural Focus of Crimean Congo Hemorrhagic Fever in the Territory of Uzbekistan, American Journal of Medicine and Medical Sciences, Vol. 14 No. 8, 2024, pp. 2057-2063. doi: 10.5923/j.ajmms.20241408.24.
1. Introduction
Currently, Crimean Congo hemorrhagic fever (CCHF), among newly emerging and returning human viral infections, is an urgentx health problem in a number of countries around the world, where outbreaks have been noted and sporadic cases of this disease have been reported. Despite the fact that the incidence of CCHF is significantly inferior to other infectious diseases, the high mortality rate (up to 30% - 50%), severe clinical course, the risk of nosocomial and family outbreaks, the sporadic or outbreak nature of the epidemic process and the social significance of the disease determine the need to study state of natural CCHF foci [5]. The established distribution area of the CCHF virus covers a vast territory of Africa, Asia, the Middle East, southern and eastern Europe, Transcaucasia and the south of the European part of Russia, the northern border of the CCHF virus distribution area does not exceed 48°N. Between 1944 and 2016, the incidence of CCHF was observed in more than 30 countries in the region. The main factor in the circulation of CCHF virus should be considered the thermal conditions necessary for the vector population [3]. The distribution area of the CCHF virus practically coincides with the habitat of ticks of the genus Hyalomma, which are the main carriers of the virus. [12]. The main reservoir of the virus in nature and the source of infection is about 27 species and subspecies of ixodid ticks. Human infection occurs through the bites of ixodid ticks, through direct contact with a patient (medical workers and members of their families often fall ill) and through the blood of infected animals (during slaughter and cutting of carcasses) [1].Over the past ten years, the highest incidence of CCHF has been observed in Russia, Turkey and Iran, where more than 50 patients with CCHF are identified annually [12,20]. Sporadic incidence is recorded in Afghanistan, Pakistan, India, Iraq, Oman, and Georgia. Introduced cases of CCHF into non-enzootic territories have been noted: to Germany from Bulgaria (2001) and Afghanistan (2009, 2012), to France from Senegal (2004), to the UK from Afghanistan (2012) and Bulgaria (2014) [4,15].The relevance and need for knowledge in the field of epizootological and epidemiological features of CCHF is dictated by the fact that the territory of Uzbekistan has long been an endemic region [2,7].Activation of natural CCHF foci with epidemic manifestations necessitates an in-depth study of a number of issues regarding the epizootology and epidemiology of this disease, which is of great importance for improving and differential approach to planning and carrying out preventive and anti-epidemic measures, which determined the purpose of our study.The purpose of the work is to study the biostructure of natural CCHF foci, the level of natural infection of rodents and ticks with the CCHF virus in order to determine the risk of population infection with the virus and predict changes in the activity of natural foci.
2. Materials and Methods
The presented work contains the results of complex studies carried out in epidemiological seasons for the period from 1988 to 2023 at the bases of the Research Institute of Virology, the Republican Center for Plague Prevention and the Center for the Development of Professional Qualifications of Medical Workers of the Ministry of Health of the Republic of Uzbekistan.Material for virological, serological, molecular genetic and zooparasitological studies was collected during expeditions. In accordance with the assigned tasks, in the process of carrying out this work, 29 expeditionary trips and business trips lasting from 5 to 45 days (278 days in total) were carried out with the participation and under the guidance of the authors of this scientific work. Biological material for laboratory studies was collected in accordance with generally accepted methodological recommendations [17,21,22].Each collection was provided with a label with numbers that made it possible to establish the date, geographical location, place of collection, characteristics of the biotope, type of ticks and rodents. All collected ticks were grouped according to the method of collection (from humans, wild, domestic mammals, “for the flag”, etc.) and pools were formed for laboratory research, taking into account the fatness, species, sex, age of ticks, host type and the nature of the biotope.Over the entire analyzed period, 6244 wild rodents were collected and examined, as well as 2961 pools of mite suspensions (about 35,000 specimens). A total of 22,683 studies were conducted (10,952 serological, 1,124 molecular genetic and 396 virological).Virological methods (infection of VERO E-6 cell culture, newborn white outbred mice - NBM), molecular genetic (polymerase chain reaction - PCR), serological (enzyme-linked immunosorbent assay - ELISA, agar gel immunodiffusion test - AGID, indirect hemagglutination - IHA) were used) [9,13,14,16,19]. When diagnosing IHA, we used the erythrocyte immunoglobulin diagnosticum (EIGD) for the CCHF virus [6,16].
3. Results and Discussion
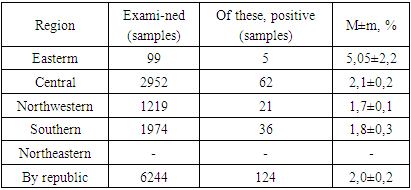
It is known that with the help of an epizootological examination of a territory it is possible to assess the epidemiological significance (i.e. the possibility and nature of the influence on the epidemic process) of biocenotic, entomological, socio-economic factors, as well as factors of the geographical environment in which parasitic systems of the epidemic process exist [8,10].Due to differences in natural and socio-economic conditions, the interaction of humans, animals and the environment with pathogens of natural focal infections occurs differently in different regions. In this regard, we divided the territory of the Republic of Uzbekistan, according to climatic-geographical and socio-economic conditions, into the Eastern, North-Eastern, Central, Southern and North-Western regions [11].The natural focus of the disease is an area of the geographical landscape with a certain biogeocenosis. Biogeocenosis is characterized by the presence of a biotope and biocenoses: carriers of the pathogen and donors of the pathogen for blood-sucking arthropods, which become carriers of this pathogen to susceptible animals. The continuous epizootic process ensures the existence of a natural focus. The epizootic process in CCHF is continuous and operates under certain conditions of the geographic environment, that is, within the boundaries of different types of outbreaks. Therefore, we conducted studies to study the biostructure of natural CCHF foci, which allows us to determine the degree of risk of infection of the population and predict changes in the degree of activity of foci [8,10,16,22,23].It has been established that wild rodents are a reservoir of the CCHF virus in natural foci and feed the pre-imaginal phases of development of many species of ixodid ticks [8,22].Due to the fact that the main reservoir of the CCHF virus in nature is wild rodents, we examined small rodents in some geographical regions of Uzbekistan for the presence of the CCHF virus antigen, identifying the types of affected rodents that can characterize the intensity of the epizootic process (Table 1).Table 1. Results of laboratory tests of rodent organ samples for the presence of CCHF virus antigen
 |
| |
|
As can be seen from the table, during the analyzed period, 5 (5.05 ± 2.2%) out of 99 samples of rodent organs in the Eastern region, out of 2952 samples from the Central region, 62 (2.1 ± 0.2%), out of 1219 samples from the North-West region 21 (1.7±0.1%), out of 1974 samples from the Southern region in 36 (1.8±0.09%), CCHF virus antigen was detected in out of 1974 samples from the Southern region in 36 (1.8±0.09%), and 1 strain of the virus was also isolated. In the North-Eastern region, rodents have not been tested for the virus. Materials collected from the Eastern and Central regions were not studied by virological methods due to the fact that the virus genome was not detected when they were studied using real-time PCR.The data obtained indicate varying degrees of exposure of rodents to the CCHF virus. The lowest degree of infection of rodents by the CCHF virus was detected in the Northwestern (1.7%) and Southern (1.8%) regions, the highest degree of infection was found in the Eastern region (5.05%) and the Central region occupies an intermediate place (2.1%).The diversity and abundance of rodent species, and the degree of their infestation is an important factor in determining the intensity of the epizootic process regarding CCHF in a given region [6,10]. Based on this, we studied the species composition, background species and the degree of infestation of rodents. Identification of species and determination of the degree of their infection shows that in the studied regions the reservoir of the virus is different species of rodents.In the Eastern region, according to our research, the antigen of the CCHF virus was detected in great gerbil (Rhombomus opimus) and midday jird (Meriones meridianus) and in the Tamarisk jird (Meriones tamariscinus). In the Central region, those affected by CCHF virus included the great gerbil, midday jird, house mouse (Mus musculus), Libyan jird (Meriones libycus) and other rodents. In the Southern region, CCHF virus infection was detected in the Libyan jird, the midday jird and other rodents, in the house mouse, and the long-clawed ground squirrel (Spermophilosis leptodactilus). In the Northwestern region, CCHF virus infection has been detected in the great gerbil, Libyan jird, midday jird, meriones and other rodents.Thus, in the studied areas, the highest prevalence of the CCHF virus was found in great gerbils, red-tailed gerbils and midday gerbils, the lowest prevalence was found in the slender-toed ground squirrel and the house mouse, and the intermediate position in terms of the degree of CCHF virus infection is occupied by the crested gerbil and other rodents (Fig. 1).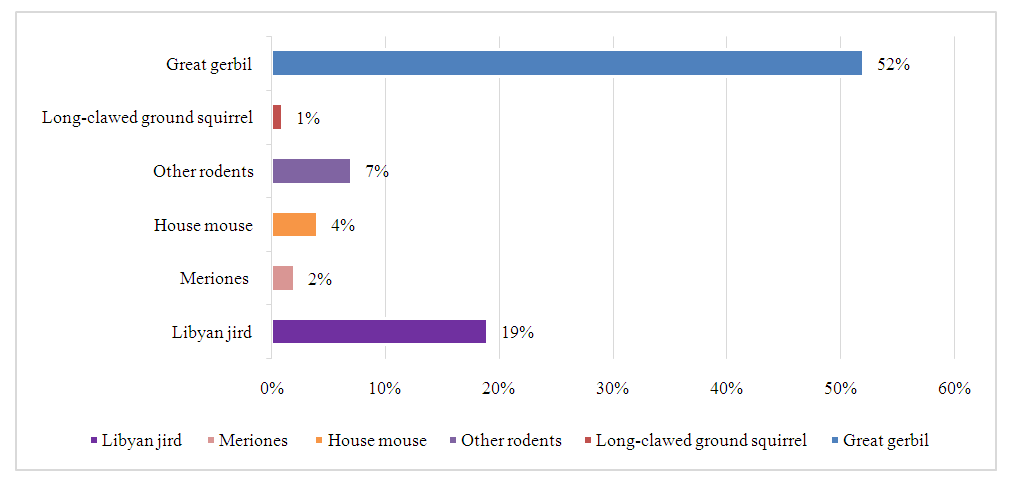 | Figure 1. Species composition of rodents affected by CCHF virus in the Republic of Uzbekistan |
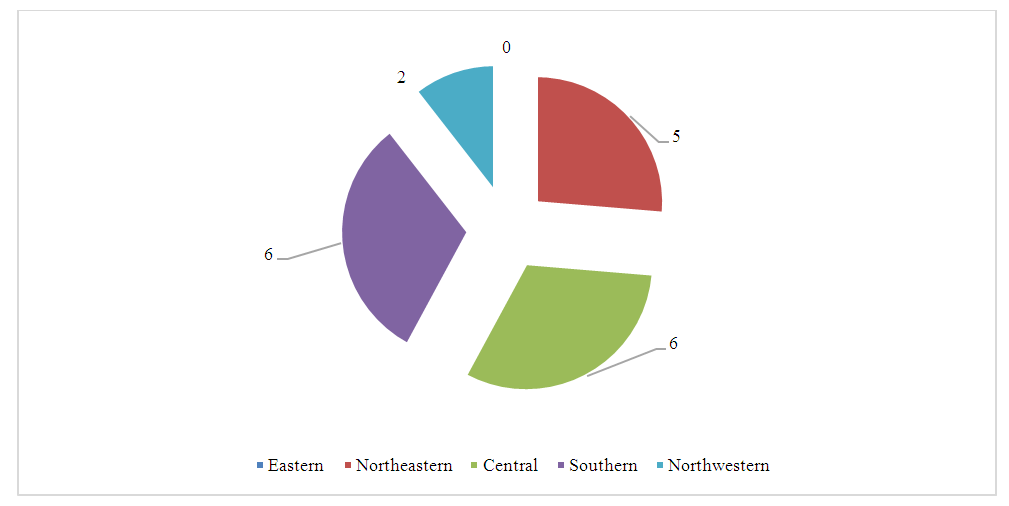
A distinctive feature of the regions is the difference in species affected by the rodent CCHF virus. Thus, the Eastern, Central and North-Western regions are characterized by infestation of great gerbils (40.0%, 83.0%, 47.0%, respectively), and the Southern region is characterized by infestation of the Libyan jird (51.0% of studies). Consequently, the indicator species of rodents for CCHF in natural foci of the studied regions are the great gerbil, the Libyan jird and the midday jird.It is known that, along with vertebrates, blood-sucking arthropods also play an important role in the preservation and spread of the CCHF virus, since transmission of the virus from an infected vertebrate animal to a healthy one occurs transmissibly through the bite of virus-carrying ticks [8,10,18,23]. Ticks are also capable of transmitting the virus transphase and transovarian to their offspring and maintaining its inter-epidemic period [8]. In order to determine the prevalence of ticks, about 35,000 thousand specimens of ticks were collected and examined by combining them into 2,961 pools, by the regions of the Republic (Fig. 2, 3).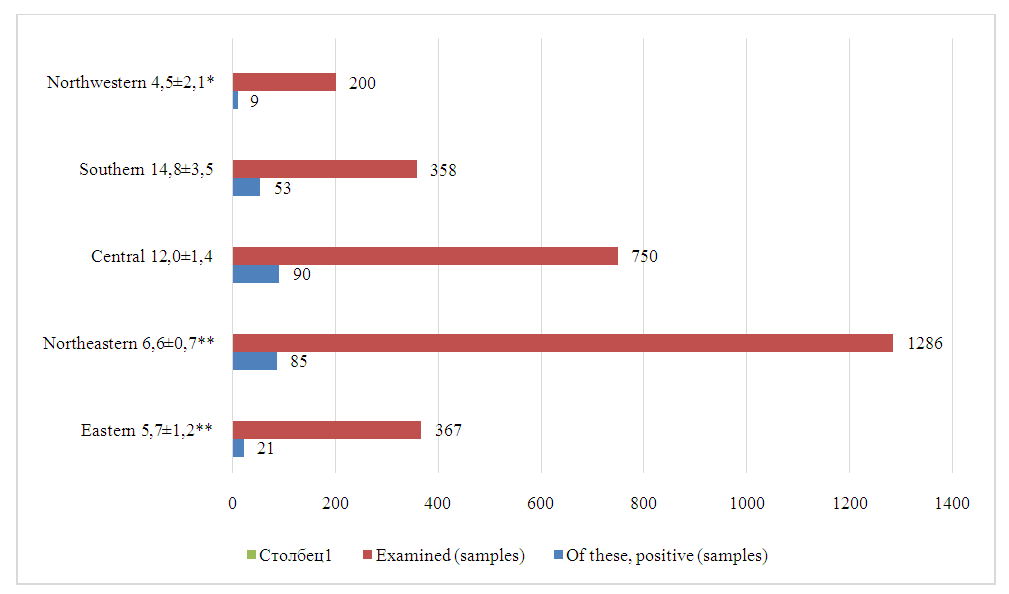 | Figure 2. Results of laboratory tests of ticks to detect CCHF virus antigen. (Note: * - Sign of significance P<0.05, ** - Sign of significance P<0.001) |
 | Figure 3. Results of laboratory tests of ticks for isolated strains of CCHF virus |
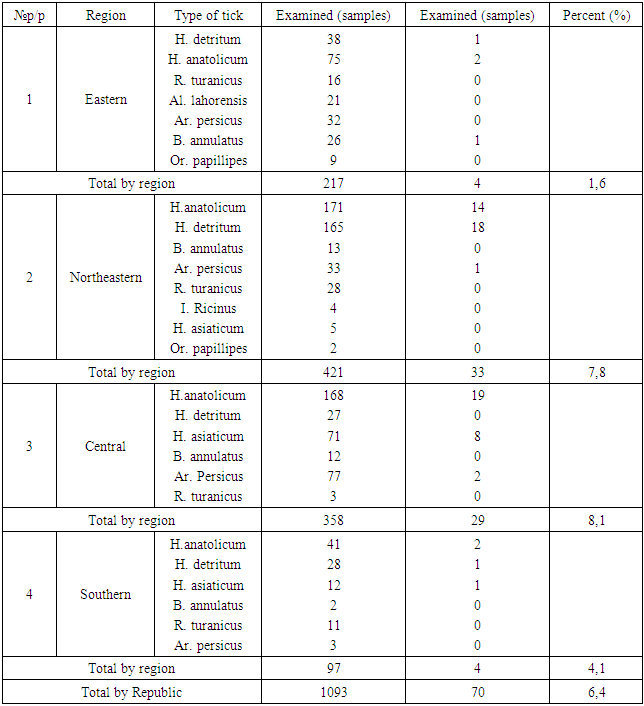
As can be seen from the figures, a higher virus prevalence when studied by serological methods was detected in the Southern - 14.8 ± 3.5% and Central - 12.0 ± 1.4% regions of the republic. The lowest virus prevalence was detected in the North-West region (4.5±2.1%). The North-Eastern - 6.6±0.5% and Eastern regions - 5.7±1.5% occupy an intermediate position.It is well known that ticks are the keepers and carriers of CCHF in nature. It was of interest to study the involvement of certain types of ticks in the carriage of the CCHF virus in the regions of the republic. For this purpose, we studied 1093 pools of mite suspensions using PCR, having previously determined their species (Table 2).Table 2. Results of a study of different types of ticks for CCHF using the PCR method
 |
| |
|
The following tick species were present in the tick collections: H. anatolicum, H. detritum, H. asiaticum, B. calcaratus, R. turanicus, Ar. persicus, Al. lahorensis, Or. papillipes, I. ricinus. The eastern region was represented by 7 species of ticks, the total number of studied pools was 217, and in 4 of them the genome of the CCHF virus was detected - they were represented by the tick species H. anatolicum - two pools, H. detritum and B. annulatus, one pool each. In the North-Eastern region, ticks are represented by 8 species. A total of 421 tick pools were studied in the region, and the genome of the CCHF virus was detected in 33 of them. Of these, 18 positive pools are represented by mites of the species H. detritum, H. anatolicum - 14 pools and Ar. Persicus 1 pool. In the Central region, 358 pools were studied; they are represented by 6 species of ticks. The CCHF virus genome was detected in 29 pools, of which 19 were H. anatolicum, 8 were H. asiaticum, and 2 were Ar. persicus. In the Southern region, the collected ticks were represented by 6 species. 97 pools were studied, of which the CCHF virus genome was identified in 4. 2 positive pools are represented by mites of the species H. anatolicum and one each by H. detritum and H. asiaticum.As can be seen from the data in Table 2, in the Eastern region, the dominant species in our collections were 6 species of mites H. detritum, H. anatolicum, R. turanicus, Al. lahorensis, B. annulatus and Ar. persicus. But the ticks infected with CCHF virus were H. anatolicum, H. detritum and B. annulatus.In the North-Eastern region, the following were prevalent in our collections: H. anatolicum, H. detritum, R. turanicus, B. annulatus and Ar. persicus. The ticks infected with CCHF virus were H. anatolicum, H. detritum and Ar. persicus. Spontaneous infection was detected in the first two species. In the Central region, the dominant species were: H. anatolicum, H. detritum, H. asiaticum, B. annulatus and Ar. persicus. A high percentage of infection was detected in H. anatolicum and H. asiaticum ticks, a lower percentage in Ar. persicus. In the Southern region, the species encountered were: H. anatolicum, H. detritum, H. asiaticum and R. turanicus. The most infected species with CCHF virus were ticks of the species H. anatolicum and were equally infected with H. detritum and H. asiaticum.As can be seen from the above data, the highest infection with the CCHF virus when studied using the PCR method was detected in the North-Eastern (7.8%) and Central (8.1%) regions. A lower (4.1%) percentage of infection was detected in the Southern region and the lowest (1.06%) in the Eastern region.In the republic as a whole, tick infection with the CCHF virus was represented by five species: H. anatolicum (53%), H. detritum (29%), H. asiaticum (13%), Ar. persicus (4%) and B. annulatus (1.0%) (Fig. 4).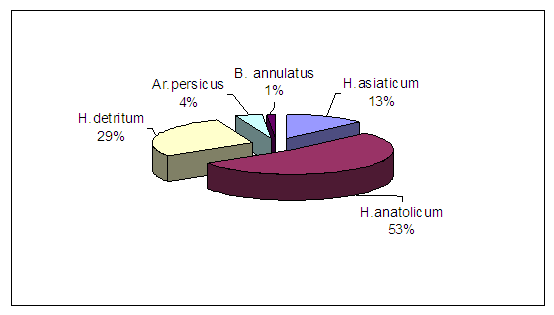 | Figure 4. Species composition of ticks infected with CCHF virus in the Republic of Uzbekistan |
Thus, the most numerous species of ticks during the study period in the Eastern region are H. anatolicum, H. detritum, B. calcaratus, Ar. persicus and R. turanicus, and the infected species were H. anatolicum, H. detritum and B. annulatus; In the North-Eastern region - H. anatolicum, H. detritum, Ar. persicus, R. turanicus and B. annulatus and the infected species were H. anatolicum, H. detritum and Ar. Persicus; In the Central - H. anatolicum, Ar. persicus, H. asiaticum, H. detritum and B. annulatus, and the infected species were H. anatolicum, H. asiaticum and Ar. Persicus; In the South with H. anatolicum, H. detritum, H. asiaticum and R. turanicus, and the infected species were H. anatolicum, H. detritum and H. asiaticum. The most common tick species in the republic are H. anatolicum, H. detritum, Ar. persicus, H. asiaticum, R. turanicus and B. annulatus.
4. Conclusions
A high level of CCHF virus infection in common tick species may indicate a high degree of danger from contact with them for farm animals and people. These indicators are prerequisites for the maturation of the epizootic process with the subsequent transition to epidemic manifestation.The authors confirm that there is no conflict of financial/non-financial interests related to the writing of the article.
References
| [1] | Antonov A.V., Belova M.V., Boyko E.A. About the results of monitoring natural focal viral infections on the territory of the Krasnodar Territory and the Republic of Adygea. // National priorities of Russia. 2021. No. 3 (42). pp. 90-93. |
| [2] | A. Nematov, N. Komilov. Study history of Crimean Congo hemorrhagic fever in Uzbekistan // Scientific and practical journal of the sanitary-epidemiological peace and public health committee of the Republic of Uzbekistan. – T., 2023. No. 2. 16-14 p. |
| [3] | Barkhash A.V. Genetic predisposition of humans and laboratory animals to various infections transmitted by ixodid ticks. / Molecular genetics, microbiology and virology. 2022. T. 40. No. 2. p. 3-13. |
| [4] | Butenko, A.M. Incidence of Crimean hemorrhagic fever in Europe, Africa and Asia (1943-2012) / A.M. Butenko, I.N. Trusova // Epidemiology and infectious diseases. - 2013. - No. 5. - p. 46-48. |
| [5] | Volynkina A.S., Tkachenko N.O., Maletskaya O.V. and others. Crimean hemorrhagic fever: epidemiological and epizootological situation in the Russian Federation in 2022, incidence forecast for 2023 // Problems of especially dangerous infections. - 2023. - No. 2. - p. 6-12. |
| [6] | Gaidamovich S.Ya., Klisenko G.A. Shanoyan N.K. Indirect hemagglutination reaction with viruses of the CCHF-Congo group // Issue. virusol. - 1974. -№6.-p. 705-708. |
| [7] | Komilov N.O. Improving epidemiological surveillance of Crimean-Congo hemorrhagic fever using geographic information systems // Journal of Theoretical and Clinical Medicine. - Tashkent, 2009. - No. 4. - pp. 66-69. |
| [8] | Kulichenko, A.N. Crimean hemorrhagic fever in Eurasia in the 21st century: epidemiological aspects / A.N. Kulichenko, O.V. Maletskaya, N.F. Vasilenko et al. // Epidemiology and infectious diseases. Current issues. - 2012. - No. 3. - p. 42-53. |
| [9] | Maletskaya, O.V. Principles of diagnostic standardization and modern features of Crimean hemorrhagic fever on the territory of the Russian Federation / O.V. Maletskaya, S.A. Shcherbakova, A.P. Beyer et al. // Problems of especially dangerous infections. - 2012. - No. 2 (112). - pp. 55-58. |
| [10] | Smirnova, S.E. Crimean-Congo hemorrhagic fever (etiology, epidemiology, laboratory diagnostics) / S.E. Smirnova. - M.: ATiSO, 2007. - 304 p. |
| [11] | Soliev A., Makhmadaliev R. The main problems of economic and social geography. Study guide. Tashkent.. 2002. - 71 p. |
| [12] | Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. Crimean- Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 2013 Oct; 100(1): 159-89. doi: 10.1016/j.antiviral.2013.07.006. Epub 2013 Jul 29. PMID: 23906741. |
| [13] | Farhadpour, F. Molecular detection of Crimean-Congo haemorrhagic fever virus in ticks collected from infested livestock populations in a New Endemic Area, South of Iran / F. Farhadpour, Z. Telmadarraiy, S. Chinikar et al. // Tropical medicine & international health. - 2016. - Vol. 21. - p. 340-347. |
| [14] | Jaaskelainen, A.J. Development and evaluation of a real-time RT-qPCR for detection of Crimean-Congo hemorrhagic fever virus representing different genotypes / A.J. Jaaskelainen, H. Kallio-Kokko, A. Ozkul et al. // Vector borne and zoonotic diseases. - 2014. - Vol. 14, N 12. - p. 870-872. |
| [15] | Mamuchishvili, N. Notes from the field: Increase in reported Crimean-Congo hemorrhagic fever cases-country of Georgia, 2014 / N. Mamuchishvili, S.J. Salyer, K. Stauffer et al. // MMWR. Morbidity and mortality weekly report. - 2015. - Vol. 64, N 8. - p. 228-229. |
| [16] | Mourya, D.T. Detection, isolation and confirmation of Crimean-Congo hemorrhagic fever virus in human, ticks and animals in Ahmadabad, India, 2010-2011 / D.T. Mourya, P.D. Yadav, A.M. Shete et al. // PLoS neglected tropical diseases. - 2012. - Vol. 6, N 5. - e1653. |
| [17] | Nurmakhanov, T. Crimean-Congo haemorrhagic fever virus in Kazakhstan (1948-2013) / T. Nurmakhanov, Y. Sansyzbaev, B. Atshabar et al. // International journal of infectious diseases. - 2015. - Vol. 38. - P. 19-23. |
| [18] | Oncu, S. Crimean-Congo hemorrhagic fever: an overview / S. Oncu // Virologica Sinica. - 2013. - Vol. 28, N 4. - P. 193-201. |
| [19] | Papa, A. Genetic detection and isolation of Crimean-Congo hemorrhagic fever virus, Kosovo, Yugoslavia / A. Papa, B. Bozovi, V. Pavlidou et al. // Emerging infectious diseases. - 2002. - Vol. 8, N 8. - P. 852-854. |
| [20] | Papa, A. Meeting report: First International Conference on Crimean-Congo hemorrhagic fever / A. Papa // Antiviral research. - 2015. - Vol. 120. - P. 57-65. |
| [21] | Shayan, S. Crimean-Congo Hemorrhagic Fever / S. Shayan, M. Bokaean, M.R. Shahrivar, S. Chinikar // Laboratory medicine. - 2015. - Vol. 46, N 3. - P. 180189. |
| [22] | Spengler, J.R. A chronological review of experimental infection studies of the role of wild animals and livestock in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus. /J.R. Spengler, A. Estrada-Pena, A.R. Garrison et al. // Antiviral Research. - 2016. - Vol. 135. - P. 31-47. |
| [23] | Vorou, R. Crimean-Congo hemorrhagic fever / R. Vorou, I.N. Pierroutsakos, H.C. Maltezou // Current opinion in infectious diseases. - 2007. -Vol. 20, N 5. - P. 495-500. |






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
