Hamza Nurmatovich Boboyev, Allaberganov Dilshod Shavkatovich, Amonov Shahrukh Rakhimovich
Department of Pathological Anatomy, Tashkent Medical Academy, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
In the period after the COVID-19 pandemic, the SARS-CoV-2 pathogen caused a nonspecific response of most organs and tissues. In particular, blockade of the APF-2 receptor in the arteries of the lung and mesentery was manifested by the development of acute dystrophic and necrotic changes, respiratory distress syndrome and acute intestinal failure, derailing the principle of blood vessel nutrition in the organs. Specific principles of morphological changes in the blood vessels of the lungs and mesentery were studied in the autopsies of those who died with the diagnosis of COVID-19. Changes in the endothelial area of the components of the blood vessel wall were studied. In the course of studies, it was found that thrombi develop as a result of severe swelling and desquamation of endotheliocytes.
Keywords:
COVID-19, Pathomorphology, Pulmonary artery, Intestinal artery, Thrombosis, Endothelium
Cite this paper: Hamza Nurmatovich Boboyev, Allaberganov Dilshod Shavkatovich, Amonov Shahrukh Rakhimovich, Pathomorphological Changes in Blood Vessels of Pulmonary and Intestinal Mesenteric Arteries in COVID-19 Infection, American Journal of Medicine and Medical Sciences, Vol. 14 No. 8, 2024, pp. 2042-2046. doi: 10.5923/j.ajmms.20241408.21.
1. Introduction
During the pandemic, death from thromboembolic complications after coronovirus infection is observed a lot (data from SSV RPAM 2021). Pulmonary edema develops as a result of interstitial edema in the alveolar wall of the lung tissue and the development of the thrombosis process in the capillaries. This, in turn, leads to many complications due to the increase of hydrostatic and oncotic, osmotic pressure in the blood vessels of the lung tissue. It is manifested in the lung tissue in the form of respiratory distress syndrome [1]. Clinical morphologically, it is manifested in the form of shortness of breath, wheezing, and continues for 6-8 hours with the formation of interstitial swellings in the alveolar walls and hyaline protein structures in the alveolar spaces. These same changes have also been found to develop in other organs [2]. However, lung tissue is one of the most dangerous organs among the vital organs, which manifests itself in the form of acute respiratory failure. Mortality in patients depends on age, gender and constitutional structure and develops differently [3]. It is precisely in the infection of COVID-19 that the blockade of the APF-2 receptor located in the vascular endothelium is manifested by dilatation of the vessel and the development of interstitial edema in the subendothelial layer and desquamation of endothelial cells.Purpose: to study the morphological changes in the pulmonary tissue and intestinal blood vessels and capillaries, alveolar walls and spaces, and to develop guidelines for clinical morphological diagnosis during coronavirus infection.
2. Materials and Methods
Research materials were obtained from 32 autopsy results of patients who died of coronavirus infection at the Republican Pathological Anatomy Center of the Ministry of Health of the Republic of Uzbekistan in 2020-2021. Gross morphological examination by hematoxylin and eosin staining, the obtained materials are fixed in glutaraldehyde, contrasted with osmium 4 oxide, and frozen in epon blocks. Then, they are cut on an ultramicrotome and the obtained micropreparations are stained with methylene blue and picrofuscin. The obtained data are examined morphologically, and clinical anamnestic analysis is carried out from the history of the disease. Then the obtained data will be statistically analyzed.
3. Research Results and Their Discussion
In our study, lung tissue and intestinal mesentery are damaged to varying degrees in small-caliber blood vessels, directly related to hemodynamics and blood rheological indicators, and it is constitutionally that the sick contingents with high body weight with COVID-19 infection are clinically and morphologically small. In caliber blood vessels, blood circulation slows down and endothelial cells are damaged due to infection [4]. If the damage is observed in the arterial blood vessels entering the organs, it ends with the development of acute ischemia and necrosis of these organs. If the damage develops in the intima of the venous blood vessels, especially in the postcapillary venules, the occurrence of hydropic dystrophy and desquamation of endothelial cells leads to the development of primary thrombi in this area (see Fig. 1). 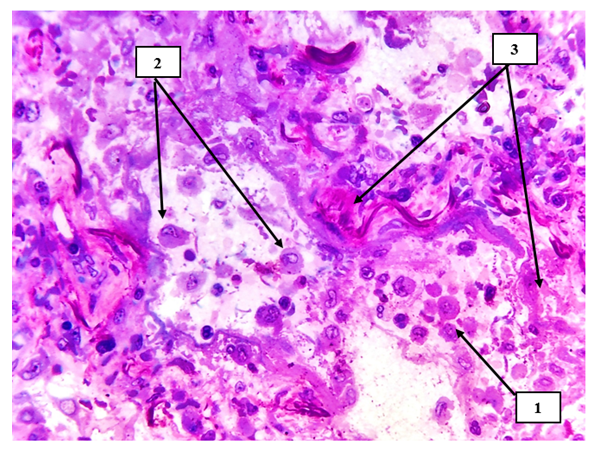 | Figure 1. Lung tissue. Macrophages with metaplastic giant cells are detected in the alveolar spaces (1), primary alveolar cells are also desquamated (2), sclerotic changes and coarse fibrous connective tissue structures are detected around small-caliber arteries (3). Semi-thin section. Stain methylene blue and picrofuscin. The size is 40x10 |
From a clinical morphological point of view, the formation of red thrombus is characterized by the fact that when it creates a sharp dynamic movement trajectory, it migrates to a large blood circulation circle and causes thromboembolic syndromes [5]. In this study, the morphological changes that occur on the surface of small caliber arteries of lung tissue damaged by SARS-CoV-2 in pulmonary blood vessels in COVID-19 were studied. It is the endothelium of muscle-type arteries with its specific features in the production and structure of secretory substances active in histologically and productively compared to large and medium-sized blood vessels (a large number of inclusions in the cytoplasm, a relatively large nucleus, high sensitivity to vasopressors and toxic substances). stand out (see Figure 2).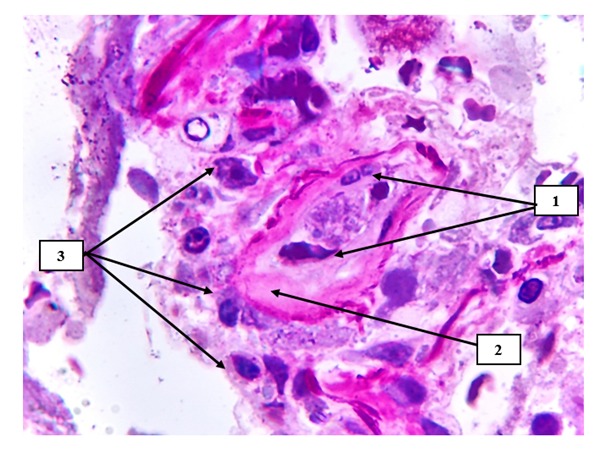 | Figure 2. Hyperchromic staining of endotheliocytes with a granular appearance on the surface of the pulmonary vascular endothelium (1), hyalinosis and pericytic area fibromatosis are detected in the vascular wall (2), chaotic arrangement of most macrophages (3). Semi-thin section. Stain methylene blue and picrofuscin. The size is 40x10 |
Under the influence of any exo and endogenous toxic substances, the formation of microthrombi in these areas due to the violation of water-salt metabolism in the endothelial cells, hydropic dystrophy and migration from the basal layer, or secondary pathomorphosis after treatment (chain migration of endothelial cells rich in giant cell inclusions and the occurrence of tissue embolism) ), characterized by a focal accumulation of macrophages and histiocytes and the development of a nodule-like formation in this area (see Figure 3).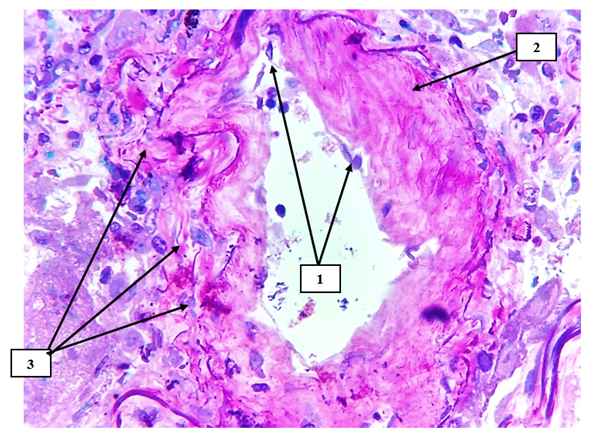 | Figure 3. Pulmonary tissue small-caliber artery. Desquamation of endotheliocytes on the surface of the arterial intima (1), hyaline uneven protein structures are detected on the vessel wall (2), irregularly migrated foci of lymphocytes and macrophages are detected on the sharply deformed surfaces of the perivascular relief (3). Semi-thin section. Stain methylene blue and picrofuscin. The size is 40x10 |
Focal desquamation of endotheliocytes on the surface of the vascular intima is considered as a factor that determines the course and duration of the process, and serves as a morphofunctional, initial factor that determines the partial or complete obliteration of the blood vessel [6].From a clinical morphological point of view, the sharp formation of sparse fibrous structures around the preserved endotheliocytes under the influence of angioprotectors (traditional membrane-stabilizing drugs ascorbic acid) that prevent damage to the vascular intima of the processes aimed at treating patients at this point, due to the adhesion of thromboplastins in these areas, due to the contractility of the blood vessel, smooth surface foci of shelf-like deformation in the intima cause an increase in ischemic processes in organs (see Fig. 4). If untreated, patients infected with COVID-19 experience massive desquamation in the area of the endothelium of the pulmonary blood vessels, which develops with blood vessel occlusion and the formation of blood clots of various degrees (see Figure 5).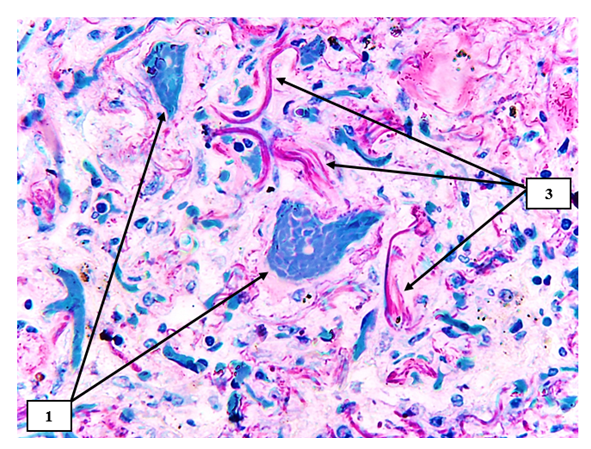 | Figure 4. Lung tissue. The histiotopography of the alveolar walls has changed, a small-caliber blood vessel with thrombosis consisting of erythrocytes is detected in the center and on the left (2). Various irregular fibrous structures with sparse fibers are identified (3). Semi-thin section. Stain methylene blue and picrofuscin |
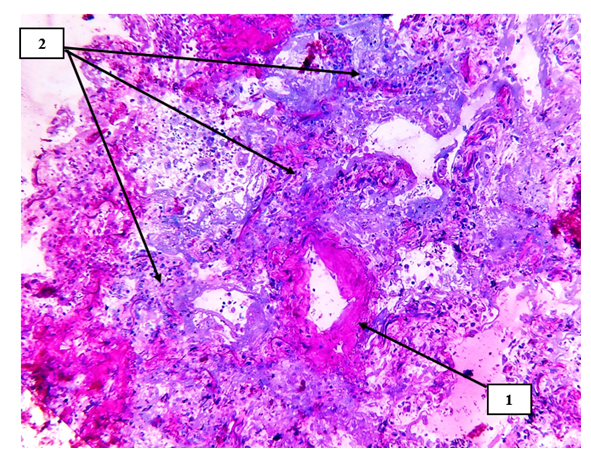 | Figure 5. Pulmonary tissue is a small-caliber blood vessel. The vascular wall is thickened, the vascular wall is sclerotized (1), the alveolar histiarchitectonics is changed, and foci of carnification after productive inflammation are identified (2). Semi-thin section. Stain methylene blue and picrofuscin |
As a result, stimulation of other vascular endothelial factor (factor of NO synthesis and re-expansion of blood vessels) in devascularized organs and foci spread and localized to the surface of blood vessels in other areas, damage to precapillary, capillary and postcapillary venules, a large number of hemorrhagic (hemorrhage per rexin type) foci continues with development [7].In the space between the vascular wall, weakly formed interstitial swellings, defragmentation in the fibrous structures and foci of hypertrophy of the muscle layer are determined. Edema and diapedesis, traumatic hemorrhage foci are detected in perivascular areas [8]. As a result, the development of these changes in the form of a large focus is confirmed by the clinical diagnosis that there is 5-15% damage in clinical morphological X-ray examinations.The presence of dark homogenous pink inclusions around the SHIFF-positive structures is manifested by the sharp accumulation of neutral mucopolysaccharides in the appearance of the SHIK-positive structures (see Figure 7). Under the influence of acidic mucopolysaccharides accumulated in the subendothelial and middle layer of mesenteric arteries, the destroyed foci of fibrous structures are identified [9]. It is found that in the case of COVID-19 infection, the endothelial cells are directly affected and the basal layer becomes inflamed and swollen under the focal desquamated cells (see Figure 8).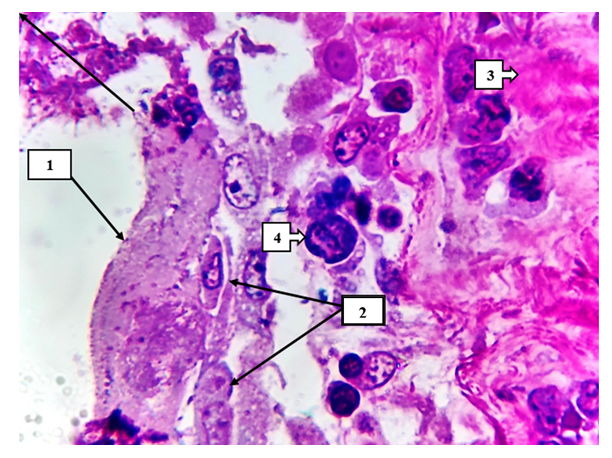 | Figure 6. On the inner surface of the alveolar wall, hyaline protein (1), dystrophic and necrobiotic changes of alveolocytes are detected (2). On the right are pink carnified foci (3). Macrophages with giant metaplasia are identified in the interval (4). Semi-thin section. Stain methylene blue and picrofuscin |
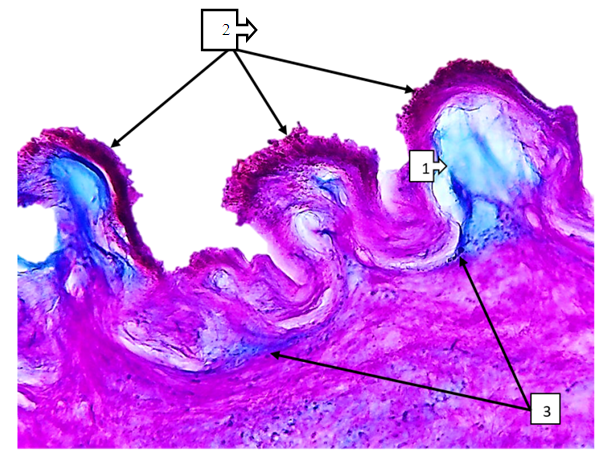 | Figure 7. Superior mesenteric artery in COVID-19. In the subendothelial space, there is a sharp accumulation of ShIFF-positive structures and mucoid discharge (1), and on the surface of the intima layer, thrombus aggregation is detected in a large and small granular form (2). The trajectory of the basal layer varies in thickness and appears thickened (3). Paint SHIFF paint. The size is 60x10 |
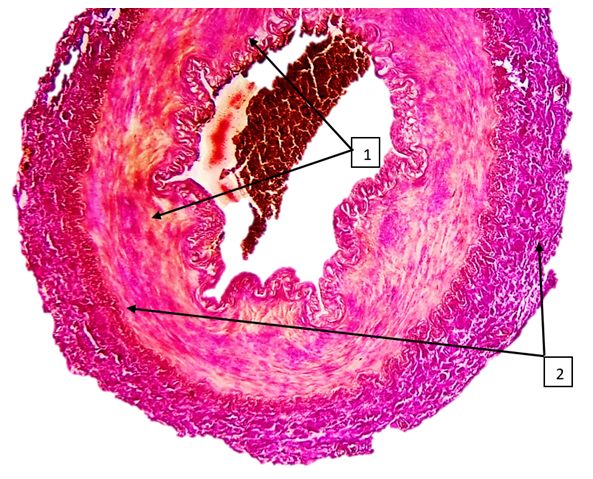 | Figure 8. Superior mesenteric artery in COVID-19. An uneven joule of fuchsinophilic fibers is found in the middle layer, and foci of irregularly located rough collagen fibers are identified in the muscle layer (2). The paint is Van Gison paint. The size is 10x10 |
As a result, the occurrence of the thrombogenesis process on damaged surfaces develops in certain periods. This is followed by the occurrence of tumors in the subendothelial layer, the destruction of fibrous structures in the areas of accumulation of acidic glycosaminoglycans, and the violation of the histioarchitectonics of the vascular wall. Schiff staining reveals irregularly formed collagen fibers between the subendothelial layer and the muscle layer. This, in turn, confirms the existence of sharply proliferative active foci of fibroblasts in these areas.It is noteworthy that the clinical morphodynamic development of lung tissue and intestinal mesentery in patients with COVID-19 infection develops approximately 1-5 days after the occurrence of the above vascular injuries, and the patients do not show organ failure (in the form of shortness of breath, shortness of breath, tachypnea) in the classical way of the process. It will not be possible to prospectively assess specific stages of development. In most clinical examinations, large red wet lungs are detected only after formation [10]. Microscopically, these changes culminate in the appearance of hyaline protein structures in the alveolar walls, the appearance of atelectasis and carnified foci in functionally damaged areas of the alveolar walls, and lead to pneumosclerosis. It is the massive angiosclerosis and obturation in the blood vessels that causes pulmonary hypertension within the small blood circulation and causes the death of the patient.Therefore, the degree of damage to the endothelium of blood vessels during the infection of COVID-19 depends on the duration of the disease, the gender and constitutional aspects of the organism, and has different manifestations. The main principle of its clinical morphological aspects is the development of thrombus and macrophage reactions in place of the displaced endotheliocytes on the surface of the intima, and the occurrence of deformationally enlarged segments due to the narrowing of the vascular space and stimulation of NO secretion in these areas is characterized by the occurrence of hemodynamic disturbances in the organs.
4. Conclusions
1. Vacuolar dystrophy and desquamation of the endothelial cells of the vascular wall occur in COVID-19.2. It is determined that the migration of endothelial cells in the walls of blood vessels, which are rich in muscle fibers, is more frequent than in blood vessels of elastic type.3. It is characterized by the development of interstitial swelling in the subendothelial layer of blood vessels of the muscular type, and the sharp narrowing of the vascular space in the contracted state of the muscles and the occurrence of acute ischemic necrosis in the organs fed by this blood vessel.4. From a clinical morphological point of view, the deformation of small-caliber blood vessels, the surrounding of narrowed vascular spaces with tissue and cell components with thromboplastins is manifested in the transient ischemic or infarcted form as a result of acute vascular occlusion.5. Carnification of lung tissue and metaplasia of interstitial macrophages due to productive inflammation of lung tissue in interstitial pneumonia after COVID-19. These changes increase the likelihood of developing tumor diseases in the future.6. In COVID-19, the increase of Schiff-positive structures in the wall of the renal artery in various degrees leads to the development of interstitial edema and changes in the vascular relief. Changes in the vascular relief lead to changes in the folds of the endothelial surface, and mechanical damage of the endothelium under the influence of turbulent flow on the surfaces of large folds is an important factor for the formation of thrombus.
References
| [1] | Babkina A.S. et al. Morphological changes in the brain in COVID-19 // General Reanimatology. - 2021. - Vol. 17. - No. 3. - P. 4-15. |
| [2] | Donduray E.A. et al. Characteristics of COVID-19 in children: the first experience of working in a St. Petersburg hospital // Journal of Infectology. - 2020. - Vol. 12. - No. 3. - P. 56-63. |
| [3] | Fedorov D.N. et al. Morphological and immunohistochemical characteristics of changes in the lymph nodes of the bronchopulmonary group in patients with a new coronavirus infection COVID-19 (according to the results of autopsy studies) // Almanac of Clinical Medicine. - 2020. - Vol. 48. - No. S1. |
| [4] | Savchenko S.V. et al. Morphological changes in the heart and blood vessels in a new coronavirus infection (COVID-19) // Bulletin of Forensic Medicine. - 2021. - Vol. 10. - No. 2. - P. 40-44. |
| [5] | Zabolotskaya F.G. et al. Pathological anatomy of the lungs in a new coronavirus infection (COVID-19). Preliminary analysis of autopsy studies // Clinical practice. - 2020. - Vol. 11. No. 2. |
| [6] | Litvinov A.S. et al. Clinical and morphological parallels of lung and kidney damage in COVID-19 // Nephrology. - 2020. - Vol. 24. - No. 5. - P. 97-107. |
| [7] | Vorobyova O.V., Lastochkin A.V. Pathomorphological changes in organs in COVID-19 // Infection and immunity. - 2020. - Vol. 10. - No. 3. |
| [8] | Samsonova I.V. et al. Pathomorphology of COVID-19 based on 15 autopsies // Bulletin of the Vitebsk State Medical University. - 2020. - Vol. 19. - No. 3. |
| [9] | Zairatyants O.V. et al. Pathological anatomy of COVID-19: experience of 2000 autopsies // Forensic medicine. - 2020. - Vol. 6. - No. 4. |
| [10] | Bondarev O.I. and others. Pathomorphological changes in organs with a combination of a new coronavirus infection (covid-19) and pneumoconiosis in workers of the coal industry of Kuzbass // Medicine in Kuzbass. - 2020. - V. 19. - No. 4. -P. 17-25. |
| [11] | Dujardin RWG, Hilderink BN, Haksteen WE, Middeldorp S, Vlaar APJ, Thachil J, Müller MCA, Juffermans NP. Biomarkers for the prediction of venous thromboembolism in critically ill COVID-19 patients. Thromb Res. 2020; 196: 308–12. |
| [12] | Tsaplin S, Schastlivtsev I, Zhuravlev S, Barinov V, Lobastov K, Caprini JA. The original and modified Caprini score equally predicts venous thromboembolism in COVID-19 patients. J Vasc Surg Venous Lymphat Disord. 2021; 9(6): 1371–81. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML






