-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(8): 1957-1961
doi:10.5923/j.ajmms.20241408.01
Received: Jul. 17, 2024; Accepted: Jul. 30, 2024; Published: Aug. 2, 2024

Sarcopenic Obesity: National Features in Cirrhosis of the Liver of Viral Etiology in Uzbekistan
Musabaev E. I., Abdieva R. M., Umurzakov B. K.
Research Institute of Virology, Republican Specialized Scientific and Practical Medical Center of Epidemiology, Microbiology, Infectious and Parasitic Diseases, Tashkent City, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background and aim: Sarcopenia can occur in both malnourished and obese patients. Sarcopenic obesity is excessive accumulation of adipose tissue combined with loss of muscle mass and strength. The aim of the study was to study the combination of sarcopenia in overweight patients with cirrhosis of the liver of viral etiology. Subjects and Methods: The selection of patients for the study was carried out from the 4th quarter of 2019 to the 1st quarter of 2020 and from 2021-2023 on the basis of the clinic of the Research Institute of Virology of the RSSPMCEMIPD in Tashkent. The criteria for inclusion of patients in the study were - presence of cirrhosis of viral HBV, HDV, HCV etiology of class A, B and C according to Child-Pugh; age from 18 to 60 years. The criteria for exclusion of patients from the study were patient's refusal from the study and liver cirrhosis of non-viral etiology. Results: According to the results of the study, the height of the patients ranged from 189 to 160 cm in males, with a mean of 174.54±5.87 cm (95% CI 173.3-175.8 cm). In females, the height ranged from 178 to 140 cm, with a mean of 162.73±6.06 cm (95% CI 161.2-164.3 cm). BMI showed insignificant changes among males and females depending on Child-Pugh grade. When determining the relationship between lumbar musculoskeletal index (SMI) and dynamometry, it was revealed that in Child-Pugh class A these features correlate weakly positively, r (25) = 0.45, p = 0.03. In Child-Pugh class B, the correlation was also weakly positive, r (32) = 0.49, p = 0.006. In Child-Pugh class C, the correlation is moderately positive, r (14) = 0.52, p = 0.08. In the course of the study, we were interested in the presence of sarcopenia in normal-weight and overweight patients depending on the Child-Pugh classes. Sarcopenia was assessed by dynamometry in 141 patients. The results in all groups were not statistically significant. Further, sarcopenia was assessed by lumbar musculoskeletal index in 71 patients. In class A of 25 patients, it was equally unreliable in both groups in 20% of cases (p=0.2); in class B - among 32 patients, it was significantly found in 9% of patients with overweight and in 44% with normal body weight (p=0.01); in class C – among 14 patients reliably in 8% and 54% (p=0.03) cases, respectively. Conclusions: Based on the findings, patients with normal body weight are more likely to have sarcopenia as cirrhosis progresses. It can be assumed that the negative results in this group of patients are associated with a strict diet with a restriction of the protein component in the diet and malnutrition. We could not estimate the percentage of muscle and fat tissue, due to the presence of edema and ascites. Those patients who did not follow a strict diet did not have sarcopenia. Despite the presence or absence of sarcopenia in patients with cirrhosis in both groups (normal weight and overweight), there was a progressive decrease in muscle strength. In the course of the study, it became known that until now, all patients have been limited in motor activity and physical activity, regardless of the stage of cirrhosis and the severity of the general condition. Such restrictive measures contribute to the development of presarcopenia or full-blown sarcopenia.
Keywords: Sarcopenic obesity, Liver cirrhosis, Dynamometry, Lumbar musculoskeletal index in liver cirrhosis
Cite this paper: Musabaev E. I., Abdieva R. M., Umurzakov B. K., Sarcopenic Obesity: National Features in Cirrhosis of the Liver of Viral Etiology in Uzbekistan, American Journal of Medicine and Medical Sciences, Vol. 14 No. 8, 2024, pp. 1957-1961. doi: 10.5923/j.ajmms.20241408.01.
1. Introduction
- Liver disease accounts for approximately 2 million deaths worldwide each year, with approximately half of them due to complications related to liver cirrhosis [1,2]. Cirrhosis of the liver can lead to many complications ending in high mortality [3], and according to the World Health Organization, in 2015, most deaths from viral hepatitis B (HBV) and hepatitis C virus (HCV), were caused by chronic liver disease with 720,000 deaths due to cirrhosis [4], showing a growing trend and imposing a high medical and economic burden. [5]. In the Republic of Uzbekistan, cirrhosis of the liver is more often caused by a combination of viral hepatitis B (HBV) and D (HDV), as a result of which degenerative changes in liver tissue are accelerated and the process quickly turns into decompensation.The liver is the second largest organ in the human body [6] and the main organ of metabolism [7]. Its functional integrity is essential for the supply and inter-organ circulation of macronutrients (proteins, fats, and carbohydrates) and their metabolism [8,9]. Nutrition plays an important role in the treatment of cirrhosis of the liver and its complications [10].Malnutrition is very common in patients with cirrhosis, occurring in at least 50% to 90% of patients, and is particularly high among patients with decompensated cirrhosis, with more than one-fifth of patients with compensated cirrhosis [10,11,12,13]. The term "sarcopenia" includes the loss of muscle mass, strength, and function. It is a major component of malnutrition and adversely affects clinical outcomes such as susceptibility to infections, progression of hepatic encephalopathy, and ascites. Reduces the quality of life of patients. It is also an independent predictor of reduced survival in patients on the waiting list for liver transplantation and in those who have undergone transplantation [14]. Survival is lower in patients with sarcopenic cirrhosis prior to liver transplantation, while after transplantation, increased length of hospital stay, longer ICU stay, and longer intubation time have been reported compared to patients without sarcopenia [15,16,17].The mechanisms of sarcopenia in cirrhosis are multifactorial, complex, and not fully understood [2,18]. These include decreased nutrient intake (both energy and protein), intestinal indigestion, and/or malabsorption and hypercatabolism [19,20,21]. Frequent fasting states and external factors such as alcohol, infections, and medications further contribute to malnutrition in patients with cirrhosis [19]. Leading to muscle wasting, changes in protein turnover, energy utilization, hormonal and metabolic changes, increased myostatin expression by reactive oxygen species, and inflammatory cytokines [7,22,23]. Although the pathogenesis of sarcopenia in cirrhosis of the liver is poorly understood, a number of proposed mechanisms representing an imbalance between muscle breakdown and formation have previously been described [22]. Muscle mass is maintained by a balance between protein synthesis, protein breakdown, and regenerative capacity. In cirrhosis, reduced energy intake further exacerbates impaired muscle biosynthesis and increased muscle proteolysis caused by low glycogen stores, leading to an increased need for gluconeogenesis [24,25,26]. There are several factors associated with sarcopenia in cirrhosis, including hyperammonemia, low testosterone, decreased human growth hormone (GH), and high endotoxin levels [24].The presence of sarcopenia is assessed by several indicators: assessment of nutrition with intervention in the patient's lifestyle, anthropometric findings, presence of fluid retention, measurement of shoulder muscle circumference and thickness of the subcutaneous fat fold, manual dynamometry, CT/MRI to measure the cross-sectional area of the abdominal skeletal muscles at the level of the third lumbar vertebra. Whole-body X-ray absorptiometry (DEXA) and tetrapolar bioelectrical impedance analysis (BIA) can be used if there is no fluid retention in the body of patients with cirrhosis of the liver [27]. Sarcopenia can also occur in obese patients, but due to the coexistence of obesity, it may go unnoticed. Sarcopene obesity is an excess accumulation of adipose tissue combined with a loss of muscle mass and strength [28].Aim of the work: The aim of the study was to study the combination of sarcopenia in overweight patients with cirrhosis of the liver of viral etiology.
2. Patients and Methods
- The study was conducted at the clinic of the Research Institute of Virology from the 4th quarter of 2019 to the 1st quarter of 2020; 2021-2023. The criteria for inclusion in this study: 1. Presence of cirrhosis of viral HBV, HDV, HCV etiology of class "A", "B" and "C" according to Child-Pugh. 2. Age from 18 to 60 years. The criteria for exclusion in this study: 1. Patient's refusal from the study. 2. Liver cirrhosis of non-viral etiology. Ethical aspect: all patients were informed in advance about the study and included in it with their consent. Thorough history taking: personal, current and past history. Clinical Examination: The objective status of the patient, anthropometric data, and dynamometry were evaluated. ECG. Laboratory investigations: General and biochemical blood tests were carried out on an autoanalyzer with a study of liver and kidney functions. ELISA and PCR of blood for markers of viral hepatitis HBs Ag, anti HCV, anti HDV antibodies. Radiological investigation: Ultrasound, chest X-ray, CT with lumbar musculoskeletal index (SMI) calculation. The total number of patients examined is 141. Of these, 51 patients with cirrhosis of the liver of class "A", 54- Class "B" and 36- Class "C" according to Child-Pugh. In the course of the study, an analysis was carried out based on anthropometric data: height, body mass index. Body mass index (BMI) was assessed by the WHO as underweight, normal, overweight and obese. In a statistical comparison of the study groups, indicators exceeding normal BMI values were combined into one overweight group. To determine the reliability of BMI between groups in men and women, Fisher's exact test (p<0.05) was used. Of the total number of patients, 73 patients had their lumbar musculoskeletal index (SMI) measured and dynamometry performed. To determine the relationship (SMI) and dynamometry, the Pearson correlation coefficient was used. Due to the presence of emissions, 2 patients were removed, so the data were evaluated in 71 patients.In the course of the study, the presence of sarcopenia was assessed in patients with normal body weight and with excess body weight. Sarcopenia was assessed by dynamometry (in 141 patients) and lumbar musculoskeletal index (in 73 patients). To determine the reliability of BMI between groups, Fisher's exact test (p<0.05) was used.
3. Results
- According to the results of the study, the height of men ranged from 189 to 160 cm, with an average value of 174.54±5.87 cm (95% CI 173.3-175.8 cm). Females range in height from 178 to 140 cm, with an average of 162.73±6.06 cm (95% CI 161.2-164.3 cm). BMI in Child-Pugh class "A" was assessed in 29 men and 22 women. Normal body weight was observed in 14 men (in 48% of cases) and in 7 women (in 32% of cases). Increased body weight in 15 men (52%) and 16 women (73%). The result is not significant: p=0.26 (p<0.05). Child-Pugh's Class B consisted of 33 men and 21 women. Of these, 24 men (73%) and 11 women (35%) were of normal body weight; 9 men (27%) and 7 women (21%) were overweight. The result is not significant: p=0.53 (<0.05). Child-Pugh's Class C consisted of 20 men and 16 women. Of these, 7 men (35%) and 10 women (62%) were of normal body weight; 9 men (45%) and 5 women (31%) were overweight. The result is not significant: p=0.28 (p<0.05). When determining the relationship between lumbar musculoskeletal index (SMI) and dynamometry, it was revealed that in Child-Pugh class "A" these features correlate weakly positively, r (25) = 0.45, p = 0.03. In Child-Pugh class B, the correlation was also weakly positive, r (32) = 0.49, p = 0.006. In Child-Pugh class C, the correlation is moderately positive, r (14)=0.52, p=0.08 (Histogram)In the study, sarcopenia was assessed by dynamometry in 141 patients. In Child-Pugh class "A" among 39 patients, it was found in 26% with overweight, and with normal body weight in 13% (p=0.2); in class "B" out of 51 patients - in 14% and 43% of cases (p=0.23); in class "C" out of 31 patients - in 32% and 39% of cases (p=1). Therefore, the results in all groups are not statistically significant. Further, sarcopenia was assessed by lumbar musculoskeletal index in 71 patients. In class "A" of 25 patients, it was equally unreliable in both groups in 20% of cases (p=0.2); in class "B" - among 32 patients, it was significantly found in 9% of patients with overweight and in 44% with normal body weight (p=0.01); in class "C" – among 14 patients reliably in 8% and 54% (p=0.03) cases, respectively.
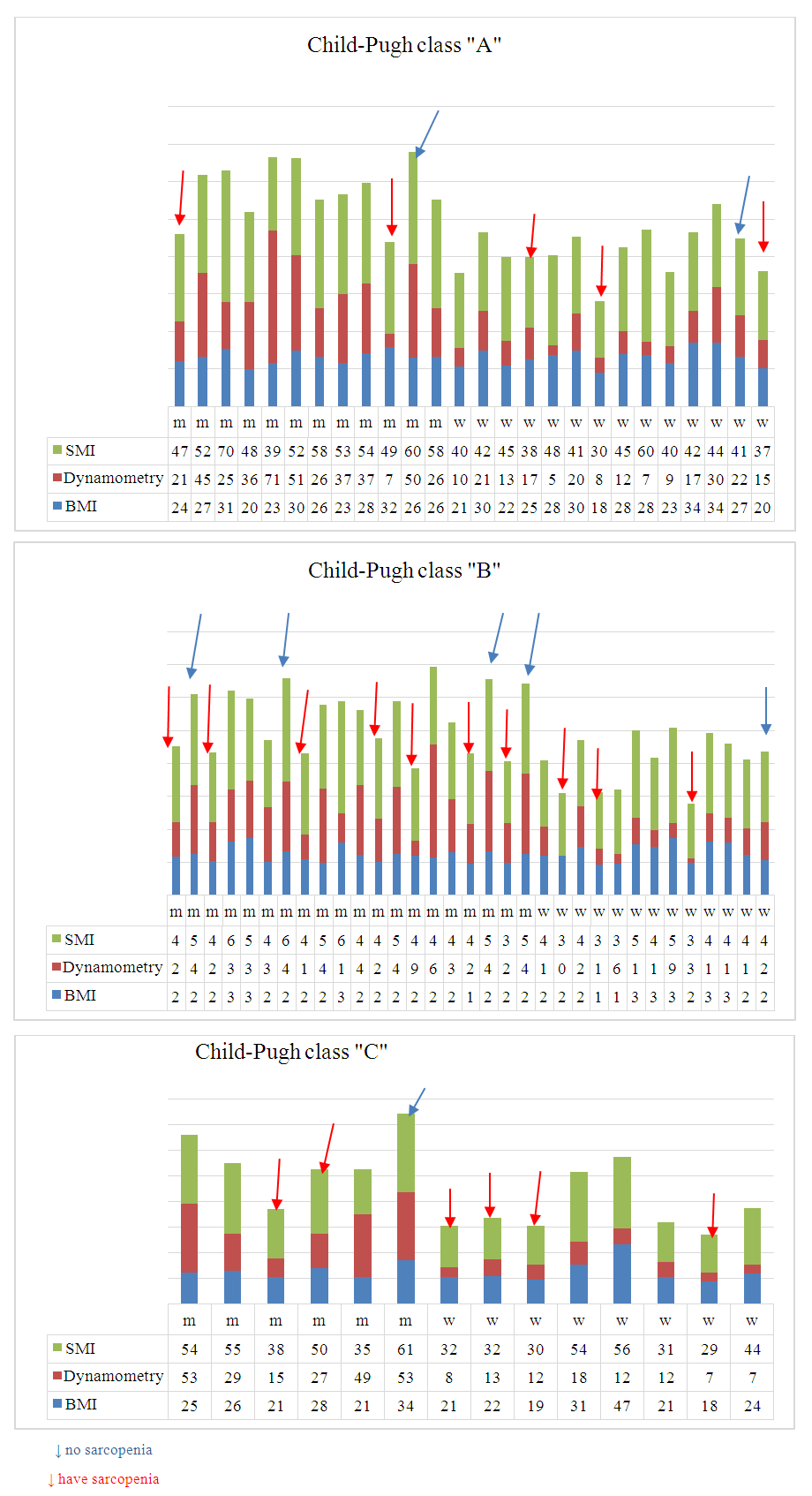 | Figure 1 |
4. Discussion and Conclusions
- Based on the data obtained, the average height of patients in Uzbekistan is more consistent with European indicators than in Asia. BMI rates for men and women in different Child-Pugh classes are not statistically significant. The strength of the correlation between SMI and dynamometry increased significantly positively from weak in Class "A" and "B" to moderate in Class "C" according to Child-Pugh. Evaluation of sarcopenia in overweight and normal patients using dynamometry did not show reliable results. In assessing SMI, sarcopenia was significantly more common in patients with normal body weight than in overweight patients. Based on the findings, patients with normal body weight are more likely to have sarcopenia as cirrhosis progresses. It can be assumed that the negative results in this group of patients are associated with a strict diet with a restriction of the protein component in the diet and malnutrition. We could not estimate the percentage of muscle and fat tissue, due to the presence of edema and ascites. Those patients who did not follow a strict diet did not have sarcopenia. Despite the presence or absence of sarcopenia in patients with cirrhosis in both groups (normal weight and overweight), there was a progressive decrease in muscle strength. In the course of the study, it became known that until now, all patients have been limited in motor activity and physical activity, regardless of the stage of cirrhosis and the severity of the general condition. Such restrictive measures contribute to the development of presarcopenia or full-blown sarcopenia.The national cuisine of Uzbekistan is diverse, high in calories and nutritious. Therefore, it is not necessary to limit patients in food, but to adapt their intake according to their general condition. Encourage physical activity in a way that takes into account the interests of patients. If there is sarcopenia and ascites, then a set of exercises should be performed while sitting or lying in bed. This study should be continued in order to assess how much the quality of life of patients will change, to find more effective methods of screening for sarcopenia.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML