-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(7): 1948-1950
doi:10.5923/j.ajmms.20241407.46
Received: Jul. 10, 2024; Accepted: Jul. 25, 2024; Published: Jul. 26, 2024

Features of Collagen Metabolism in Compact and Cancellous Bone Tissue in Rats with Alloxan Diabetes
Mirzaev Aziz Kakhhorovich1, Yusupov Shukhrat Adurasulovich2, Butolin Evgeny Germanovich3, Olga Vladimirovna Danilova4, Telang Sahir Prasenjit5, Khalikov Kakhor Mirzaevich6
1Independent Applicant of Samarkand State Medical University, Uzbekistan
2Doctor of Medical Sciences, Head of the Department of Pediatric Surgery No1, Uzbekistan
3Doctor of Medical Sciences, Head of the Department of Clinical Biochemistry and Laboratory Diagnostics of the Faculty of Advanced Training and Professional Retraining of the Izhevsk State Medical Academy, Uzbekistan
4Department of Biochemistry, Candidate of Medical Sciences of the Izhevsk State Medical Academy, Uzbekistan
5Assistant in the Department of Pediatrics Surgery No.1, Samarkand State Medical University, Uzbekistan
6Head of the Department of Biochemistry Samarkand State Medical University, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

One of the chronic complications of diabetes mellitus is diabetic osteopathy, which manifests itself from moderate osteoporosis to spontaneous fractures of long bones [13]. The mechanisms of development of diabetic osteopathy in patients with diabetes have not been sufficiently studied [3]. At the same time, in the pathogenesis of the development of osteopathy, the following has been established: a) a decrease in the production of collagen and alkaline phosphatase by osteoblasts, caused by a deficiency of insulin, necessary for the formation of organic and inorganic bone matrix; b) indirectly, through insulin-like growth factors, a decrease in stimulation of osteoblasts; c) increased bone resorption by osteoclasts caused by the accumulation of glycation products [1].
Keywords: Alloxan diabetes
Cite this paper: Mirzaev Aziz Kakhhorovich, Yusupov Shukhrat Adurasulovich, Butolin Evgeny Germanovich, Olga Vladimirovna Danilova, Telang Sahir Prasenjit, Khalikov Kakhor Mirzaevich, Features of Collagen Metabolism in Compact and Cancellous Bone Tissue in Rats with Alloxan Diabetes, American Journal of Medicine and Medical Sciences, Vol. 14 No. 7, 2024, pp. 1948-1950. doi: 10.5923/j.ajmms.20241407.46.
1. Introduction
- In patients with diabetes mellitus, the activity of biochemical markers of bone metabolism changes significantly. Thus, the synthesis of type 1 bone collagen is significantly reduced, derivatives of transverse collagen fibers specific to bones and cartilage tissue - pyridinoline and deoxypyridinoline, as well as hydroxyproline, an amino acid exclusively contained in collagen proteins, are intensively excreted in the urine [9]. Similar data were obtained in experimental diabetes [8]. However, one must take into account the fact that hydroxyproline is formed during the breakdown of not only bone collagen, but also collagen that is localized in other tissues. Thus, changes in the level of hydroxyproline should be considered as an indirect sign of the development of metabolic changes in the bones.Bone is a constantly renewed tissue and there are two main metabolic processes continuously present in it - bone formation and bone resorption. Substances associated with these two processes are considered markers of bone collagen [2,4]. These markers, in addition to those listed above, include amino- and carboxyl-terminal properties of type 1 procollagen: PINP and PICP - markers of bone formation. Amino- and carboxy-terminal telopeptides of type 1 collagen associated with cross-links: β-CrossLaps are markers of bone resorption [11,14].Diabetes can cause the development of irreversible complications in bone tissue; Further study of the pathogenetic mechanisms of osteopathy is necessary, including in experimental models of diabetes mellitus. Models of experimental diabetes in laboratory animals are varied, including the alloxan model, which is widely used. Numerous studies have established that the use of alloxan in rats causes a condition similar in manifestations to insulin-dependent diabetes mellitus: persistent hyperglycemia and hypoinsulinemia [1,15].
2. Materials and Methods
- Experiments were carried out on 48 white outbred male rats weighing 180-220 grams in compliance with the principles of humane treatment of animals set out in the Declaration of Helsinki (2000). The animals were kept on a standard vivarium diet with free access to water.In rats of the experimental group (38 animals), diabetes mellitus was induced by a single subcutaneous injection of alloxan tetrahydrate (Sigma-Aldrich, USA) at a dose of 170 mg/kg body weight [5]. The reproduction of diabetes was monitored by the following indicators: the level of glycemia in blood plasma using the glucose oxidase method and the level of glycated hemoglobin in whole blood (NycoCard-HbA1c on a NycoCard Reader 11 reflectometer, USA). The animals were taken out of the experiment on days 14 and 28 under short-term ether anesthesia. The mortality rate during the experiment was 36%.In homogenates of compact bone tissue (femoral diaphysis) and cancellous bone tissue (body of the 2nd lumbar vertebra) the following was determined: the amount of total collagen [14], PINP (ELISA, ELISA; Cloud-Clone Corp., USA), beta-CrossLaps (ELISA, ELISA; IDS SERUM CrossLaps, UK), activity of collagenolytic enzymes (CA) according to E. Schalinatus modified (12). The amount of SA was expressed in millimoles of hydroxyproline per 1 kg of dry tissue mass (mmol/kg), PINP and b-CrossLaps - in picograms per 1 ml of homogenate supernatant (pg/ml), CA - in micromoles of hydroxyproline per 1 g of protein per hour (μmol /g*h).Statistical processing of the obtained data was carried out using the Statistica software package from Stat Soft. In the sample groups, the median (Me) and interquartile range (25%; 75%) were determined. The statistical significance of differences between groups was assessed using the Wilcoxon-Mann-Whitney U test with a critical level of 0.05.
3. Results and Discussion
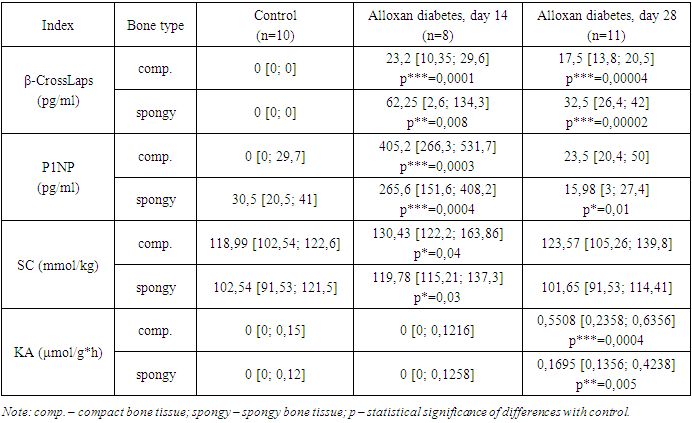
- Administration of alloxan to animals caused the development of hyperglycemia and an increase in the level of glycated hemoglobin. Thus, by the 14th day of the experiment, the content of glucose and glycated hemoglobin increased by 89.7% (p = 0.0004) and 25.3% (p = 0.0006), respectively; by the 28th day of the study, the studied indicators were 152.1% (p = 0.0001) and 148.8% (p = 0.001), respectively, which suggests the development of diabetes in experimental animals.The state of collagen metabolism is determined by the processes of accumulation and breakdown of this protein. The prevalence of synthetic processes of bone tissue collagen is indicated by an increase in the content of SA and the level of P1NP [6,11]. The intensity of the breakdown of the main protein of connective tissue is characterized by an increase in the concentration of b-CrossLaps [11,14], the level of CA and a decrease in the amount of SC [8].By the 14th day of the study, in the diaphysis of the femur and the body of the 2nd lumbar vertebra, the level of the bone collagen resorption marker b-CrossLaps increased from 0 to 23.2 pg/ml (p = 0.0001) and from 0 to 62.25 pg/ml (p=0.008), respectively, compared to the control. On the 28th day of the experiment, the content of the studied markers decreased and amounted to 17.5 pg/ml (p = 0.00004) and 32.5 pg/ml (p = 0.00002), respectively. During the same period of alloxan diabetes, an increase in the level of KA was detected from 0 to 0.5508 µmol/g*h (p=0.0004) in compact bone and from 0 to 0.1695 µmol/g*h (p=0.00004) – in spongy bone tissue. There were no significant significant differences in the SC indicator compared to the control, either in the diaphysis of the femur or in the body of the 2nd lumbar vertebra (Table 1).
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML