Yusupova G. K.1, Fayzullaeva N. Ya.2, Raufov A. A.2, Ashurova D. T.1
1Tashkent Pediatric Medical Institute, Uzbekistan
2Institute of Human Immunology and Genomics, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Background: Acute respiratory viral infections (SARS) are a significant cause of acute carditis, especially in children, contributing to a substantial burden on healthcare systems. Despite relative epidemiological stability, these infections still dominate infectious pathology, with each influenza epidemic correlating with increased cases of acute myocarditis. Methods: This study aimed to investigate innate and adaptive immunity, cytokine status, and autoimmune markers in children with non-traumatic carditis and dilated cardiomyopathy (DCM). A total of 106 children with cardiac pathology (non-rheumatic carditis and DCM) and 20 control subjects underwent comprehensive clinical examinations, including laboratory and instrumental studies. Immunological parameters were assessed using various methods, including enzyme-linked immunosorbent assay (ELISA) and rosette reaction. Results: Children with non-rheumatic carditis and DCM showed significant alterations in immune profiles compared to the control group. T-lymphocyte imbalance, including decreased T-helper cells and suppressed suppressor activity, was observed. B lymphocytes and serum immunoglobulins, particularly IgM and IgA, were elevated. There was increased killer cell activity and T-proliferating cells, with cytokine analysis revealing elevated IL-4 and decreased IFN-γ levels. Antinuclear antibodies and anti-single-stranded DNA antibodies were also elevated, suggesting autoimmune involvement. Conclusion: Children with non-traumatic carditis and DCM exhibit distinct immune alterations, including T-cell imbalance, humoral activation, increased cytotoxic activity, and cytokine dysregulation, indicating ongoing inflammation and potential autoimmune processes. These findings underscore the need for further investigation into autoimmune mechanisms and genetic predispositions in pediatric cardiac pathology.
Keywords:
Autoantibodies, Non-rheumatic carditis, DCM, Immune system, Diagnosis
Cite this paper: Yusupova G. K., Fayzullaeva N. Ya., Raufov A. A., Ashurova D. T., Immunological Markers of Non-Rheumatic Carditis and Dilated Cardiomyopathy, American Journal of Medicine and Medical Sciences, Vol. 14 No. 7, 2024, pp. 1925-1930. doi: 10.5923/j.ajmms.20241407.41.
1. Introduction
One of the main causes of acute carditis today is acute respiratory viral infections (SARS), which remain the most common and global diseases in children. Despite the relative epidemiological well-being over the past 8-10 years, they still account for 70-90% of infectious pathology and cause enormous social and economic damage [1,3,7,19]. Each influenza epidemic is accompanied by an increase in the number of cases of acute myocarditis, which determines the relevance of studying this problem [2,4,8,20].Complicated forms of SARS, which cause a severe course of the disease and determine an unfavorable prognosis, acquire the greatest importance in clinical practice [3,5,9,13].Diagnosis and treatment of cardiac pathology are one of the most pressing problems in modern medicine. In recent years, a number of researchers have made attempts to use special methods aimed at identifying inflammatory damage to the heart of infectious, allergic or autoimmune origin as a possible factor in the development of various forms of carditis in children [10,12,16,21].Autoimmune reactions underlie a wide range of both systemic and organ-specific diseases. The diversity of mechanisms for the formation of an autoimmune response currently does not allow us to fully trace all stages of the immunopathological process. However, its constant component remains the presence of organ-specific autoantibodies [11,13,17,20].Modern studies have demonstrated the ability of a number of specific autoantibodies to directly affect heart tissue, leading to myocardial dysfunction, disturbances in the bioelectrical activity of the heart and, as a consequence, to the development of carditis and myocarditis. Despite the fact that various explanations can be given for the detection of autoantibodies, there is no doubt that they serve as markers of the autoimmune process and have important diagnostic value. Antibodies to denatured (single-stranded) DNA are not specific to certain diseases and are the main components of most nuclear antibodies.Purpose of the study: to study the features of innate and adaptive immunity, cytokine status and autoimmune markers in children with non-traumatic carditis and deuteral cardiomyopathy.
2. Materials and Methods
Under our supervision were 106 children with cardiac pathology (NC, DCM) aged from 1 to 18 years (10.5 ± 1.23), who were undergoing inpatient examination and treatment in the cardiorheumatology department of the RSNPMCP clinic, from 2021 to 2023. All patients underwent a complete clinical examination, including laboratory and instrumental studies. As a comparison group, 20 children of similar age were examined.Expression of CD receptors was carried out in a rosette reaction using monoclonal antibodies of the LT series produced by Sorbent LLC.Immunological research was carried out using the method of enzyme-linked immunosorbent assay (ELISA) with the study of the content of immunoglobulins of classes A, G, M, E in the blood serum.To determine the concentrations of IL-4, IFNγ, CRP, C3, C4 in the blood serum of the study groups, a three-stage “sandwich” method was used - this is a type of three-phase ELISA.Statistical processing of the research results was carried out using the SPSS v16.0, R, PLINK and Haploview 4.2 software packages.
3. Results
A study of the subpopulation composition of T-lymphocytes showed that the level of T-helper cells was significantly reduced in the group of children with NK and DCM (23.4±1.8%, 20.1±1.3% versus 37.5±1.1%), (P<0.05). One of the characteristic features of T-cell immunodeficiency may be a decrease in the relative number of T-helper cells [6,14].Indicators of suppressor activity were lower in children with DCM - 12.7 ± 0.65% and non-rheumatic carditis - 14.3 ± 0.72% than in the control group (18.7 ± 1.15%). The reduced level of suppressor activity of the subpopulation of T lymphocytes may be associated with the movement of CD8+ cells to the site of inflammation, as well as with the basic drugs used during treatment [18]. The imbalance in the state of T lymphocyte subpopulations is dynamic in nature (Fig. 1.) [3,15].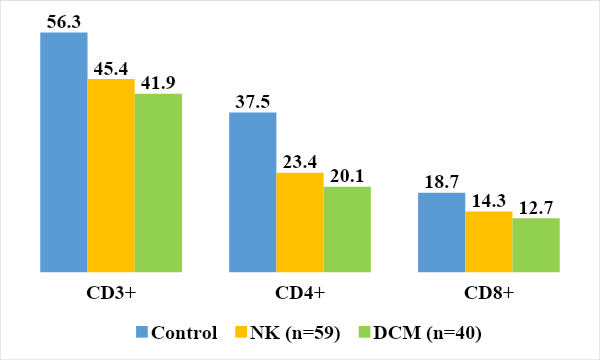 | Figure 1. Relative values of indicators of the T-immune system in the examined children |
This imbalance in the content of the subpopulation composition of T-lymphocytes was reflected in the immunoregulatory index, which was reduced in the group of sick children with NC and DCM (Fig. 2.).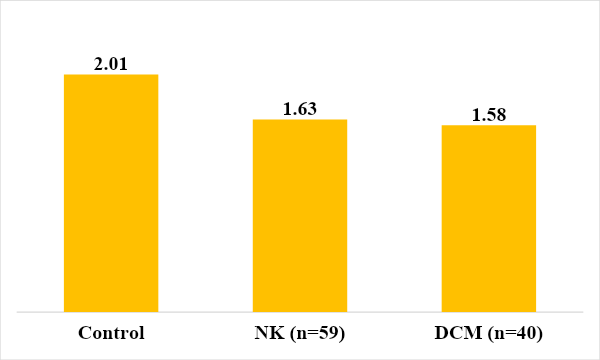 | Figure 2. Immunoregulation index in those examined |
B lymphocytes can develop an adequate immune response only with the help of T helper cells. It was found that the level of CD20+ lymphocytes tended to increase in children with DCM and NK – 25.4 ± 3.1 (Fig. 3.).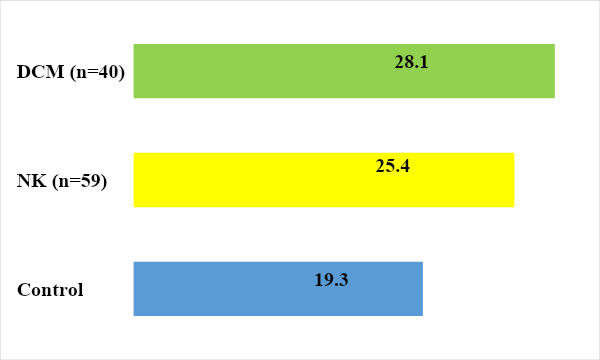 | Figure 3. Indicators of humoral immunity in those examined |
It should be noted that absolute values are labile and depend on leukocytes and lymphocytes.The results of studying the content of serum immunoglobulins in the blood of patients showed a tendency to increase the synthesis of IgG and IgM in both groups (Fig. 4). At the same time, the IgA level was significantly increased in the group of children with DCM by 1.8 times, respectively, compared to the control group 2.35 ± 0.3 (P<0.05).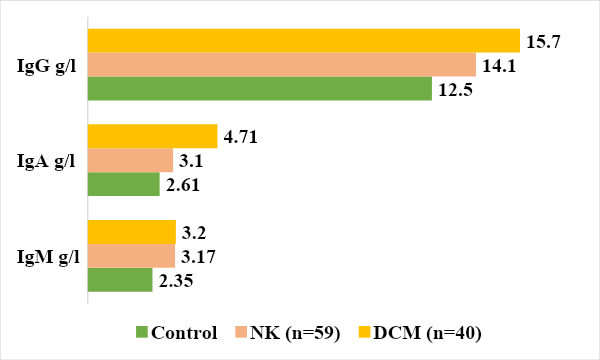 | Figure 4. Level of immunoglobulins in the examined groups |
We also studied the level of immunoglobulin E, which revealed a significant increase in its level almost twofold in children with DCM and a tendency to increase in the group with NC by 1.38 times compared to the control group (97.5±8.6 g/ l, 72.6±7.4 g/l and 52.4±5.42 g/l in the control, respectively).Next, we studied the parameters of innate immunity, such as acute phase protein CRP and natural killer cells CD16+ lymphocytes. The main function of the acute phase protein system is the removal (elimination) of foreign cells and regulation of the immune response. One of them is C-reactive protein (CRP), an acute phase protein related to nonspecific protective factors produced by liver cells [5,12]. When analyzing the results of C reactive protein in children with DCM and NC, an increase in their level was revealed in 62.8% and 54.2%, respectively. In children with DCM, the average value of C reactive protein was 7.56±2.05 mg/l, and in children with NC the average value was 6.28±1.85 mg/l, which was 2.57 and 2.14 times higher indicators of the control group (2.94±0.96 mg/l). (Fig. 5.)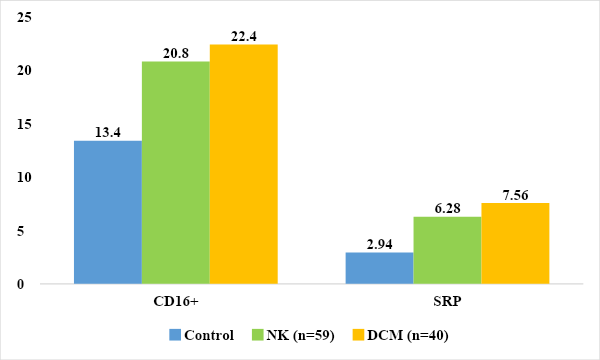 | Figure 5. Parameters of innate immunity in the examined children mg/l, % |
Analysis of the results revealed a significantly increased level of killer activity in the group of examined children with NC and DCM (22.4±1.1% 20.8±0.6% versus 13.4±0.2% in the control), (P<0.05).The complement system is a cascade of 20 proteins - blood plasma enzymes that provide an immune response in response to the interaction of an antigen with an antibody. This system is responsible for phagocytosis, destruction of foreign bacteria and supports various inflammatory reactions. Activation of the complement cascade can be carried out in the classical way, in which the stimulating factor is the interaction of an antigen with an antibody, or in an alternative way, when these factors are polysaccharides, endotoxins or immunoglobulins. Regardless of the initial stimulating factor, the final product of activation of the complement system is a complex protein capable of destroying cell membranes containing foreign antigens [4,13].The nine major components of complement are designated C1–C9. To identify disturbances in the complement system and assess its functional integrity, the determination of two components is usually carried out - C3 and C4 [2,9].The C3 component is synthesized in various tissues and organs and accounts for up to 70% of all complement proteins. It is involved in both the classical (activated by antigen complexes with IgG, IgM) and alternative activation pathways (activated by antigen complexes with IgA, IgE, Fab fragments of Ig, polysaccharide antigens of bacteria) [7,16].C3 is a key component of complement involved in ensuring nonspecific resistance (resistance) of the body to bacterial infection. Under the influence of C3, the permeability of the vascular wall increases and leukocytes move to the site of inflammation, they degranulate, resulting in the release of a large amount of biologically active substances. Fixation of the C3 component of complement on the bacterial cell wall (opsonization) leads to increased phagocytosis. In addition, the C3 component plays an important role in the development of autoimmune diseases: it is part of immune complexes. A decrease in C3 levels can lead to a weakening of the opsonizing function of the blood, phagocytosis and cytolysis [8,21].C4 is a component of the classical pathway of complement activation (the interaction of complement components with the antigen-antibody complex), which is important in the development of autoimmune diseases.The C4 component of complement is synthesized mainly in the liver, lungs and bones and is activated only through the classical pathway. It is involved in ensuring phagocytosis, neutralizing viruses and increases the permeability of the vascular wall. Its content decreases with active consumption due to activation along the classical pathway, and resistance to infectious diseases may decrease. In addition, deficiency of the C4 component is associated with a predisposition to systemic lupus erythematosus. In immune complex diseases, C4 is adsorbed on immune complexes, and its level in the blood decreases [4,11].When analyzing the levels of C3 and C4, we found a tendency for its level to increase in the group of children with NC compared to the control group (0.95 ± 0.02 g/l and 0.16 ± 0.001 g/l).In children with DCM, the level of C3 (0.45±0.02 g/l) was significantly reduced compared to the control group by 1.84 times, and in the level of C4 (0.37±0.001 g/l) no significant differences were found changes (P<0.001), (Fig. 6). A decrease in C3 with normal C4 is possibly due to the formation of autoimmune complexes and increased consumption or indicates a congenital deficiency of C3 in a group of children with dilational cardiomyopathy.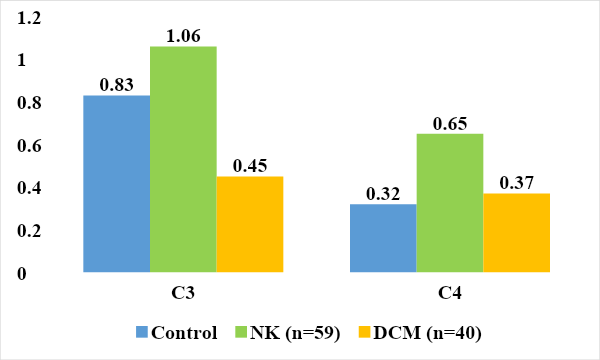 | Figure 6. Compliment component system children examined (g/l) |
When studying the results of the level of T-proliferating cells, a significant increase in its level was revealed in both groups of children (24.8 ± 3.4%, 26.7 ± 3.9%) than in the control group (16.5 ± 1.2%). And in the level of apoptotic cells, no significant differences were detected (25.9 ± 3.5%, 24.2 ± 3.1% versus 22.3 ± 2.5, respectively), (Fig. 7.)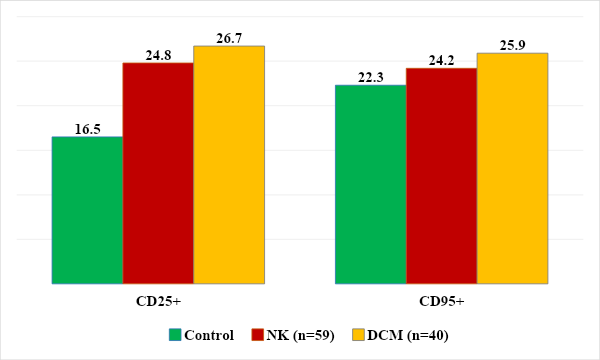 | Figure 7. Level of proliferating and apoptotic cells |
In the formation of carditis in children, immune-mediated mechanisms also play a large role, in particular, changes in cytokine status that cause immune reactions with a predominance of the inflammatory process, which allows them to be assessed as “markers” [2,7].It is known that cytokines are a group of polypeptide mediators involved in the formation and regulation of the body’s defense reactions. Studying the level of cytokines provides information about the functional activity of different types of immunocompetent cells; the severity of the inflammatory process, its transition to the systemic level and prognosis, activation of T-helper types 1 and 2 provides information about the course of the process [13].Cytokines, such as IFN-γ and IL-4, are involved in the regulation of specific immune responses by regulating the amplitude and duration of immune-inflammatory responses [16].The source of IFN-γ is activated T lymphocytes and natural killer cells. Among T lymphocytes, both cytotoxic CD8+ and helper CD4+ cells are producers of proinflammatory cytokines, but when they differentiate into Th1 and Th2, only Th1 cells retain the ability to produce proinflammatory cytokines. The most important function of proinflammatory cytokines is to mediate the relationship between lymphocytes and macrophages and participate in the regulation of the ratio of cellular and humoral components of the immune response. The proinflammatory cytokine, which is the main product of Th1 cells, reduces the secretory activity of Th2 cells [15].IFN-γ has been described as a B-cell stimulating factor because it induces B-cell proliferation. The main producers of IL-4 are class 2 T helper cells. IL-4 is synthesized by mast cells and B cell lineage cells. IL-4 reduces the functions of macrophages and their secretion of IL-1β, TNFa and IL-6, and has an anti-inflammatory effect. Thus, IL-4 is the main product of Th2 cells and stimulates their differentiation. Causes differentiation of B- and T-lymphocytes, affects the development of hematopoietic cells, macrophages, natural killer cells, basophils, which are functional antagonists of cytokines produced by Th1 cells. IL-4 promotes the development of allergic reactions and has a pronounced anti-inflammatory effect [10].The nature of anti-inflammatory cytokines in children with non-rheumatic carditis and DCM was studied. The obtained data are presented in Figure 8.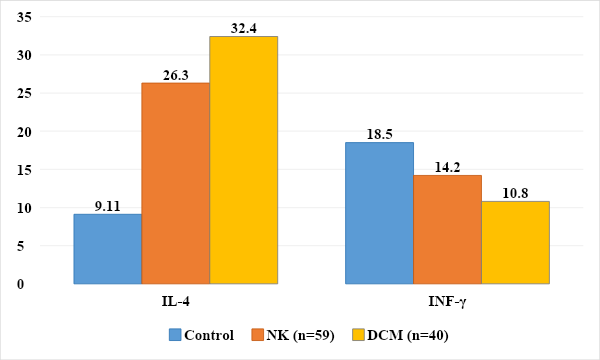 | Figure 8. Level of pro- and anti-inflammatory cytokines in the examined children, pg/ml, (P≤0.05) |
Inflammation and the type of anti-inflammatory cytokines in children with carditis were studied. Analysis of the results revealed significant differences between the secretion of cytokines in children of the control and main groups. The level of IFN-y in healthy children averaged 18.5±3.18 pg/ml, while in children with NC and DCM this figure was 14.2±3.07 pg/ml and 10.8±2.36 pg/ml. In children with DCM, the level of IFN-y was significantly lower by 1.71 times, which indicated the intensity of the inflammatory process (P≤0.05).When studying the level of IL-4 in children of the control group, it was 9.1±1.15 pg/ml, and in children with DCM and NC - 32.4±4.01 pg/ml and 26.3±3.93 pg/ml ml respectively. At the same time, the level of the anti-inflammatory cytokine IL-4 was significantly increased by 3.56 and 2.89 times (P<0.05) (Fig. 8).The identified changes in the content and ratio of pro- and anti-inflammatory cytokines in the blood serum of children with non-rheumatic carditis and cardiomyopathies confirm the persistence of the inflammatory process in cardiomyocytes.Antinuclear antibodies (ANA) and anti-ssDNA antibodies are a family of autoantibodies that bind to nuclear structures and ribonucleic/deoxyribonucleic acids and their associated proteins. They occur in more than 90% of patients with connective tissue diseases. In addition, members of this family of autoantibodies can be found in many other autoimmune, infectious, inflammatory and oncological diseases. The main mechanism for inducing the formation of ANA and sDNA appears to be an immune response against postcellular structures formed as a result of apoptosis of keratinocytes, lymphocytes and other cells, which occurs in diffuse and inflammatory connective tissue diseases. Thus, in 1994, it was shown that during the induction of apoptosis in a keratinocyte culture, the main antinuclear antibody antigens accumulate in several populations of apoptotic bodies on the cell surface. These apoptotic bodies, containing AHA and sDNA antigens, resemble viral particles in structure and are capable of inducing specific immune responses [5,17,21].Although the determination of antinuclear antibodies and single-stranded DNA in the blood is mainly used for autoimmune connective tissue diseases and inflammatory heart diseases such as non-rheumatic carditis and dilated cardiomyopathies of unknown etiology, this test can reveal the formed autoimmune orientation in combination with other clinical manifestations [6,17]. The exclusion criteria cohort for our study included autoimmune diseases, rheumatic diseases and connective tissue diseases.When analyzing the level of antinuclear antibodies, an increase was found in the group of children with NC in 25.4% (n=15) and in the group with DCM in 27.5% (n=11) with a range of variation from 6.70 to 45. 1 and from 7.09 to 99.8 standard units.Also, analysis of the level of antibodies to single-stranded DNA revealed an increase in its level in the group of children with NK in 18.6% (n=11) and in the group with DCM in 20% (n=8) with a range of variation from 3.25 to 13. 8 and from 4.37 to 17.5 U/ml (Fig. 9).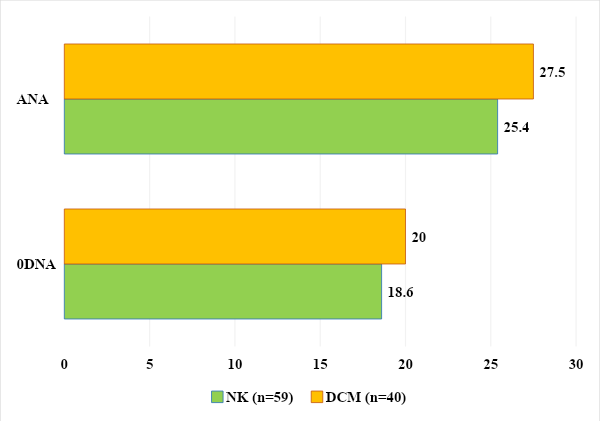 | Figure 9. Quantitative analysis of ANA and antibodies to sDNA in examined children |
4. Conclusions
Thus, the identified antinuclear antibodies and antibodies to single-stranded DNA indicate a developed autoimmune orientation in the examined children and a possible genetic predisposition to autoimmunization, triggered by previous inflammatory diseases or viral invasion. The results obtained provide the basis for further research into candidate genes of an autoimmune nature in the pathology of cardiac tissue in pediatric practice.Thus, our studies allowed us to draw the following conclusions: In the initial immune status; decrease in T-lymphocytes, helpers and suppressors and imbalance of IRI; in the humoral level, an increase in the level of B lymphocytes and serum immunoglobulins, in particular IgM; activation of killer and profiling cells; in the cytakin status, the level of IL-4 increases and the level of INF-y decreases. Also, an increase in the level of antibodies in a certain group of children with the studied nosology indicates a possible autoimmune orientation.
References
| [1] | Amdani SM, et al. Successful treatment of fulminant neonatal enteroviral myocarditis in monochorionic diamniotic twins with cardiopulmonary support, intravenous immunoglobulin and pocapavir. BMJ Case Rep. 2018; bcr-2017-224133. doi:10.1136/bcr-2017-224133. |
| [2] | Anzai A, et al. Self-reactive CD4(+) IL-3(+) T cells amplify autoimmune inflammation in myocarditis by inciting monocyte chemotaxis. J Exp Med. 2019; 216: 369–383. doi:10.1084/jem.20180471. |
| [3] | Baik SH, et al. A case of influenza associated fulminant myocarditis successfully treated with intravenous peramivir. Infect Chemother. 2015; 47: 272–277. doi:10.3947/ic.2015.47.4.272. |
| [4] | Basso C, Calabrese F, Corrado D, Thiene G. Postmortem diagnosis in sudden cardiac death victims: macroscopic, microscopic and molecular findings. Cardiovasc Res. 2001; 50: 290–300. |
| [5] | Baughman KL. Diagnosis of myocarditis: death of Dallas criteria. Circulation. 2006; 113: 593-595. doi:10.1161/CIRCULATIONAHA.105.594129. |
| [6] | Branchereau M, Burcelin R, Heymes C. The gut microbiome and heart failure: a better gut for a better heart. Rev Endocr Metab Disord. 2019; 20: 407–414. doi:10.1007/s11154-019-09519-1. |
| [7] | Amatruda C, Ceravolo GP, et al. Myocarditis in children - from infection to autoimmunity. J Biol Regul Homeost Agents. 2020; 34(4 Suppl. 2): 37-41. |
| [8] | Cuppari C, Amatruda C, Ceravolo GP, et al. Myocarditis in children - from infection to autoimmunity. J Biol Regul Homeost Agents. 2020; 34(4 Suppl. 2): 37-41. |
| [9] | Caforio A, Pankuweit S, Charron P, et al. Current state of knowledge on aetiology, diagnosis, management and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013; 34: 2636–2648. doi:10.1093/eurheartj/eht210. |
| [10] | Canter CE, Simpson KE. Diagnosis and treatment of myocarditis in children in the current era. Circulation. 2014; 129(1): 115–128. doi:10.1161/CIRCULATIONAHA.113.001372. |
| [11] | Tschöpe C, Ammirati E, Bozkurt B, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021; 18:169–193. doi:10.1038/s41569-020-00435. |
| [12] | Chang YJ, Hsiao HJ, Hsia SH, et al. Analysis of clinical parameters and echocardiography as predictors of fatal pediatric myocarditis. PLoS One. 2019; 14(3): e0214087. doi:10.1371/journal.pone.0214087. |
| [13] | Chen P, et al. Susceptibility to autoimmune myocarditis is associated with intrinsic differences in CD4(+) T cells. Clin Exp Immunol. 2012; 169:79–88. doi:10.1111/j.1365-2249.2012.04635.x. |
| [14] | Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease. J Am Coll Cardiol. 2007; 50(19):1914–1931. doi:10.1016/j.jacc.2007.09.008. |
| [15] | Fayzullaeva NY, Raufov AA. Clinical aspects of bronchial asthma and chronic obstructive pulmonary disease in adults. Academia: An International Multidisciplinary Research Journal. 2021; 11(2): 297-235. doi:10.5958/2249-7137.2021.00486.9. |
| [16] | Raufov AA, Fayzullaeva NY, Tairova SF, Atajanova NM. The relationship between vitamin D levels and obesity in patients with overlapping bronchial asthma and COPD. Chin J Ind Hyg Occup Dis. 2021; 39(7): 383. doi:10.5281/zenodo.5517628. |
| [17] | Raufov AA, Fayzullaeva NY. Clinical case of late detection of cftr gene mutation. Chin J Ind Hyg Occup Dis. 2021; 39(7): 396. doi:10.5281/zenodo.5517738. |
| [18] | Di Bella G, Pizzino F, Calabrò M, et al. New findings and the role of cardiac imaging in myocarditis and related induced cardiomyopathy. J Cardiovasc Echography. 2012; 22: 54-59. doi:10.4103/2211-4122.98016. |
| [19] | Durani Y, Egan M, Baffa J, et al. Pediatric myocarditis: presenting clinical characteristics. Am J Emerg Med. 2009; 27(8): 942–947. doi:10.1016/j.ajem.2008.07.032. |
| [20] | Tschope C, Ammirati E, Bozkurt B, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021; 18: 169–193. doi:10.1038/s41569-020-00435. |
| [21] | Chang YJ, Hsiao HJ, Hsia SH, et al. Analysis of clinical parameters and echocardiography as predictors of fatal pediatric myocarditis. PLoS One. 2019; 14(3). doi:10.1371/journal.pone.0214087. |










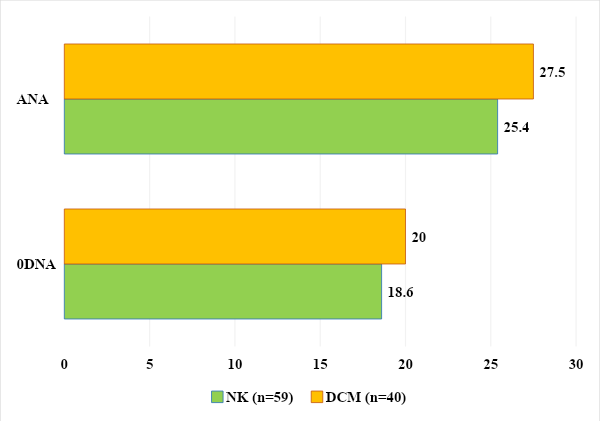
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML