-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(7): 1919-1924
doi:10.5923/j.ajmms.20241407.40
Received: Jun. 10, 2024; Accepted: Jul. 22, 2024; Published: Jul. 26, 2024

The Role of the IL-1β Gene in the Development of Pneumonia-Associated with COVID-19 in Pregnant Women
Mehrinoz Oybekjonqizi Komilova1, Shahnoza Alimjanovna Zufarova2, Kodirjon Tuxtabaevich Boboev3, Azada Sobirovna Yuldasheva4
1Basic Doctoral Student, Andijan State Medical Institute, 2-Department of Obstetrics and Gynecology, Andijan, Uzbekistan
2Doctor of Medical Sciences, Director of the Republican Population Reproductive Health Center, Tashkent, Uzbekistan
3Doctor of Medical Sciences, Professor Head of the Department of Molecular Medicine and Cell Technologies Republican Specialized Scientific and Practical Medical Center of Hematology, Tashkent, Uzbekistan
4Candidate of Medical Sciences, Associate Professor of Andijan State Medical Institute, 2-Department of Obstetrics and Gynecology, Andijan, Uzbekistan
Correspondence to: Mehrinoz Oybekjonqizi Komilova, Basic Doctoral Student, Andijan State Medical Institute, 2-Department of Obstetrics and Gynecology, Andijan, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Studying the influence of heredity, in particular, the importance of polymorphic variants of the IL-1β gene in the progress of COVID-19 infection can be useful in explaining the pathogenesis of the disease and preventing the origin of complications. The aim of the study is to analyze the association of rs1143627 polymorphism of IL-1β gene with the pathogenesis of pneumonia in women infected with COVID-19. The aggravation of the disease and the frequency of complications in pregnant and non-pregnant women infected with COVID-19 were studied in the main and control samples of the rs1143627 polymorphism of the IL-1b gene. Real-time PCR analysis of genomic DNA isolated from peripheral blood was performed. The vertical T/T genotype was statistically more common in healthy donor samples (p<0.05). Comparison of heterozygous T/C genotype in pregnant women with COVID-19 and controls did not reach significant results (p>0.05). The proportion of mutant homozygous genotype C/C was higher in pregnant women infected with COVID-19 than in the control group (20.0% and 10.5%, respectively), but statistically significant values were not obtained (p>0.05). Conclusion: the results show that the vertical T/T genotype of the IL-1b gene polymorphism rs1143627 has a protective property with high statistical confidence, the heterozygous T/C genotype is not associated with the development of coronavirus, as well as the presence of the mutant homozygous C/C genotype there is a tendency between the outbreak of the disease and the occurrence of pneumonia.
Keywords: Polymorphism, IL-1β gene, Genotype, Alleles, Odds ratio, Cytokine storm
Cite this paper: Mehrinoz Oybekjonqizi Komilova, Shahnoza Alimjanovna Zufarova, Kodirjon Tuxtabaevich Boboev, Azada Sobirovna Yuldasheva, The Role of the IL-1β Gene in the Development of Pneumonia-Associated with COVID-19 in Pregnant Women, American Journal of Medicine and Medical Sciences, Vol. 14 No. 7, 2024, pp. 1919-1924. doi: 10.5923/j.ajmms.20241407.40.
Article Outline
1. Enter
- COVID-19 is an infection that is spreading worldwide as a pandemic and poses a serious threat to people's health and life. The actuality of the problem is the fight against coronavirus, despite the introduction of vaccination, spreading the disease [1-3]. One of the most common complications in women infected with Covid-19 is the development of pneumonia, which sometimes develops without obvious clinical symptoms. Pneumonia in pregnant women poses a serious threat to both the health of the woman and the condition of the unborn fetus. Based on available epidemiologic data on the spread of novel coronavirus infection in pregnant women and the experience of other pandemics, it has been hypothesized that the course of COVID-19 in pregnant women may also vary among different populations and ethnic groups [4-7].Exacerbation of coronavirus infection usually occurs within a week after the onset of symptoms when viral titers decrease, suggesting a role of deregulated inflammatory response in the pathogenesis of disease exacerbation [8-10]. Currently, the role of the IL-1 family, more than any other cytokine family, is considered the most important, and it is well studied in the spectrum of auto-inflammatory diseases, cardiovascular diseases [11-13]. Observations show that IL-1β gene expression is low or undetectable in the absence of pathological processes, but significantly increases in the blood in disease conditions [14]. IL-1β or IL-6 genes have been hypothesized to be key pathological factors in severe COVID-19 [15] and Aggravation of COVID-19 is integrated with elevated IL-1β and IL-6 responses [10,16-18]. Furthermore, the hyperinflammation state in COVID-19 has been found to resemble some aspects of hemophagocytic lymphohistiocytosis (HLH), a condition that may be exacerbated by therapeutic blockade of IL-1β [19]. Several studies, analyzing peripheral blood mononuclear cells from patients with COVID-19, have shown a significant decrease in CD4 + T cells and CD8 + T cells and an increase in MOs in the early stage of recovery. In addition, erythrocyte sedimentation rate was higher in classical CD14 ++ Monocytes and CD14 ++ IL-1β + Monocytes with strong inflammatory gene expression [20]. Using this information, it is possible to investigate genetic factors in patients, in particular IL-1β gene polymorphisms, to develop individual treatment plans and to prevent the risk of disease complications.
2. The Purpose of the Study
- Analyze the association of rs1143627 polymorphism of IL-1β gene with the pathogenesis of pneumonia in women infected with COVID-19.
3. Materials and Research Methods
- In a scientific study, the IL-1β gene rs1143627 polymorphism was analyzed using a case-control model. The main group included 110 Uzbek women who were infected with various degrees of COVID-19 during pregnancy and were not vaccinated at that time. 36.4% of them were women aged 18-25 (40), 49.1% were women aged 26-35 (54), 14.5% were women over 36 (16). The women involved in the study were at different stages of pregnancy. The women recruited for the study were divided into two groups: subgroup A - 70 patients with a severe form of COVID-19, that is, all of them had pneumonia and respiratory symptoms (SPO2≤94, focal changes characteristic of pneumonia in X-ray analysis). Subgroup B consists of 40 pregnant women with mild viral infection with asymptomatic or general symptoms: fever, loss of appetite, general weakness. Genomic DNA preparations (n=105) from conditionally healthy donors without symptoms of COVID-19 in the anamnesis were used as material for the control group.In the anamnesis of the women taken for examination, none of them had been vaccinated against COVID-19. In order to clearly show the role of genetic polymorphisms in the pathological process and taking into account the high probability of the emergence of new strong strains of the virus and the fact that existing vaccines may not be resistant to new strains of the virus, only unvaccinated women were selected for the study.Molecular genetic testing is carried out at the Department of Molecular Medicine and Cell Technologies, on the basis of the Scientific and Practical Center of Hematology of the Republic of Uzbekistan.The examination consisted of several stages, first, venous blood samples were taken from the patients, then the DNA molecule was isolated from lymphocytes in the blood sample, polymorphisms were determined using PCR, and the results were determined. Polymorphisms were determined according to the instructions of Litekh (Russia).PCR analysis was performed according to the following amplification programs:Initial denaturation for IL-1β gene rs1143627 polymorphism - 94C0 (3 min.1 cycle), 35 amplification cycles: 94C0 (10 sec) - denaturation, 60C0 (10 min) - softening (thermal treatment), 72C0 (20 sec) - elongation and final synthesis 72 C0 (1 min. 1 cycle), kept for 10 minutes.Fragments of the analyzed PCR products (DNA) were imaged in a UV transilluminator (wavelength 310 nm) with a built-in digital camera. The results of the analysis of the fluorescent signal for each of the samples allow us to answer whether each allele is present in a heterozygous or homozygous form.The degree of association of allelic and genotypic variants was estimated by OR and RR values with 95% confidence intervals (95% CI).The statistical significance of the study was studied using the Epi info and R Microsoft office software package (2021).
4. Results
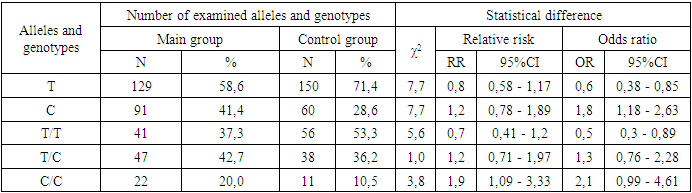
- Depending on the obtained consequences, the distribution frequencies of alleles and genotypes of rs1143627 polymorphism in the IL-1β gene were studied, and the differences between them were analyzed to identify the association with the origin of the coronavirus disease and the aggravation of the clinical condition in patients.The results of the comparative comparison showed that the percentage of the T allele was 58.6% in the main group of examined women and 71.4% in the control group, which is statistically significant in protecting against COVID-19 (χ2=7.7; p=0.01;OR=0.6; 95%CI: 0,38 - 0,85) (Table 1).
|
|
|
5. Discussion
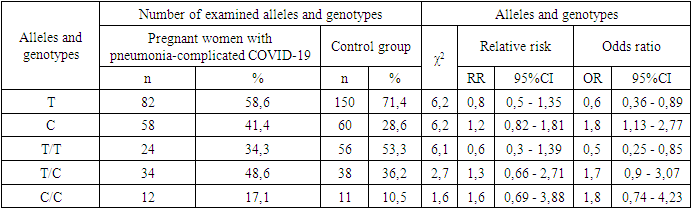
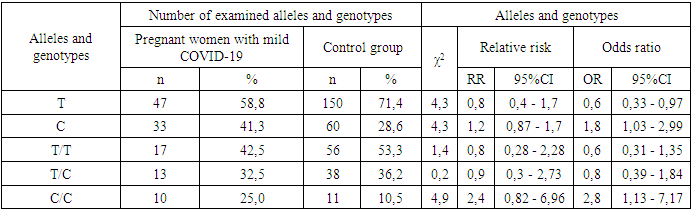
- Several studies show that interleukin IL-1βlevels are elevated in women infected with COVID-19. It is known that IL-1βfamily of cytokines plays a crucial role in triggering a cytokine storm due to uncontrolled immune responses in COVID-19 infection [21]. However, the role of this polymorphic locus in pregnant women infected with COVID-19 has not been sufficiently studied, despite the present study.During the study of the distribution of the genotypes of the rs1143627 polymorphism in the IL-1β gene for the main group of women infected with COVID-19 and the control sample, it was found that the heterozygous T/C genotype was statistically insignificant. COVID-19 (χ2=1.0; p>0.05; 95% CI: 0.71-1.97). Detection of the mutant C/C genotype was 2.1 times higher in patients than in the control group, and women with this genotype had a tendency to develop the disease (χ2=3.8; p>0.05; 95% CI: 1.09-3.33; OR=2.1; 95% CI: 0.99 - 4.61), the wild-type T/T genotype was found to be highly statistically significant in protecting against the disease, which was 37.3% in the main group of pregnant women with COVID-19 and controls group is 53.3% (χ2=5.6; p<0.05; 95% CI: 0.41–1.2) (Table 1).When analyzing the role of the IL-1β gene in the development of mild or severe forms of the disease, the heterozygous T/C genotype was statistically insignificant in the severity of the disease (p>0.05). In the group of pregnant women with a form of coronavirus complicated by pneumonia, the proportion of this genotype is 48.6% and according to the odds ratio: χ2=2.7; p>0.05; 95% CI: 0.66 - 2.71, in pregnant women with a mild form of COVID-19 it was 32.5%, χ2=0.2; p>0.05; 95% CI: 0.3 - 2.7, at the same time the percentage of T/C genotype in the control group was 36.2% (Table 2).The frequency of appearance of the homozygous mutant C/C genotype was 17.1% in pregnant women with pneumonia-associated with COVID-19, showing a tendency to increase the risk of disease complications by 1.8 times (χ2=1.6; p>0.05; OR=1.8; 95% CI: 0.69- 3.88). This genotype of the IL-1β gene is equal to 25.0% in the group of women with a mild form of viral infection and 10.5% in the control sample, and with high confidence in statistical processing, it was found that the C/C genotype increases the occurrence of this form of the disease by 2.8 times (χ2=4.9; p<0.05; OR=2.8; 95% CI: 0.82-6.96) (Table 3).Based on common parameters, the results of our study showed that the T/T genotype has a protective role against the disease (p=0.03). Also, the homozygous wild-type genotype performs a protective function in the development of the severe form of COVID-19 with obvious statistical certainty (34.3% vs. 53.3%, respectively, χ2=6.1; p<0.05; 95% CI: 0.3 - 1.39). However, statistically significant results of this genotype in protection against mild form of viral injection were not observed (25.0% and 10.5%, respectively; χ2=1.4; p>0.05; 95% CI: 0.28 - 2.28).The analysis of the literature shows that there are correlations between the development of complications of Covid-19 infection and the increase of inflammatory processes (Huang et al. 2020; Bullard et al. 2020; Zhang et al., 2020). According to them, the role of interleukins and the genes responsible for them is important in the pathogenesis of viral infection. Also, Hadjadj et al. (2020) explained the pathological processes associated with protein secretion in the disease of COVID-19 as being related to the secretion of interleukins. Based on the evidence, the IL-1 pathway has a significant antiviral effect and helps the immune system to fight viral infection [22-23]. However, based on these data, studies using interleukin therapies in COVID-19 patients have not yielded positive results, suggesting that IL-1β is not associated with disease progression (Lucy CK Bell et al., 2020). Also, IL1β levels did not predict disease progression in hospitalized patients, despite decreased cytokine activity in patients recovering from COVID-19 (Thwaites et al., 2020). The above controversies indicate the need for broader cross-national and cross-category genetic studies for early diagnosis of COVID-19 outbreaks and pneumonia.Studies worldwide have identified gender-related differences in the severity of COVID-19 between men and women as a result of genetic analysis, and observed a significantly higher risk of developing a COVID-19 outbreak in men compared to women [24]. Another study in China found that men were more susceptible to the effects of COVID-19 than women [25]. Therefore, it is appropriate to carry out genetic research in a sex-separated state. Because the clinical outcomes and complications of COVID-19 vary between countries and different ethnicities, researchers encourage genetic studies to explain this observation [26].Despite the fact that our research was studied in the contingent of pregnant women, it helps to clarify the role of genetic factors in their physiological conditions: immune system, respiratory system and other anatomo-physiological changes.
6. Summary
- According to the results of our research, IL-1β gene rs1143627 polymorphism plays an important role in preventing the development and complications of COVID-19 infection. Carriers of the T allele of this polymorphism have strong protection against the disease, while patients with the recessive C allele have a more severe form of viral infection. The analysis of genotypes showed that the homozygous T/T genotype has a statistically higher protective function, but the presence of the heterozygous T/C genotype does not play a significant role in the outbreak of the coronavirus disease. The homozygous mutant C/C genotype has a tendency to increase the risk of disease progression and pneumonia in COVID-19. Genetic testing is one of the most harmless and effective methods among pregnant women, early prediction of the disease outbreak helps to prevent the development of adverse pathological conditions in the mother and the fetus.Also, understanding the mechanisms and mediators involved in the cytokine storm may help to find effective ways to treat and combat SARS-COV-2 infection.Currently, the only way to protect against COVID-19 remains vaccination, however, more extensive, international research is needed to understand the pathogenesis of the virus and to prepare for additional risks such as the emergence of new, more virulent strains.
ACKNOWLEDGEMENTS
- We would like to thank all the co-authors who helped to conduct our study and prepare the manuscript, who participated in data collection, and all patient groups who participated in the investigation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML