-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(7): 1862-1868
doi:10.5923/j.ajmms.20241407.30
Received: Jun. 25, 2024; Accepted: Jul. 19, 2024; Published: Jul. 20, 2024

Clinical, Hormonal and Imaging Features of Inactive Pituitary Adenomas with Recurrent and Stable Course
Kholova Dilorom Sharifovna, Halimova Zamira Yusufovna
Republican Specialized Scientific and Practical Medical Center of Endocrinology named after. Academician Y.Kh. Turakulov, Tashkent, Republic of Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

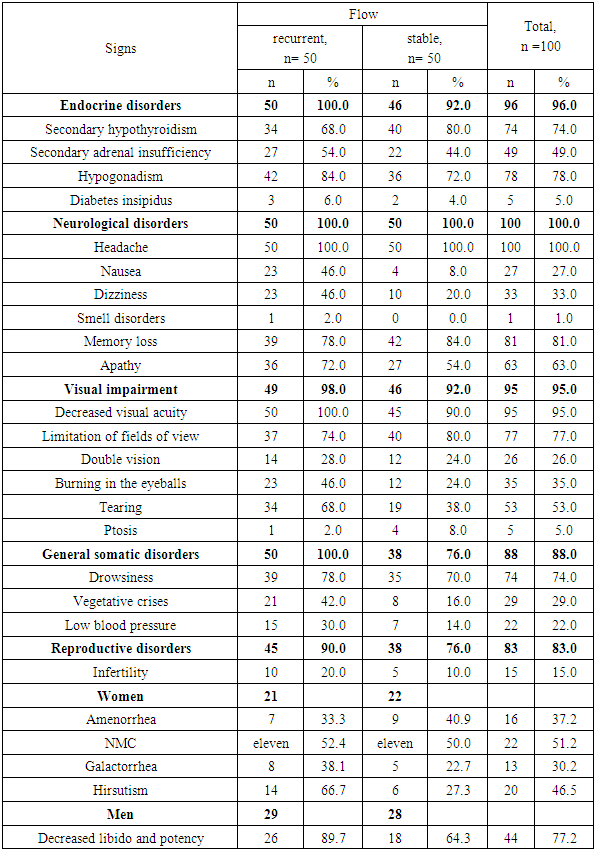
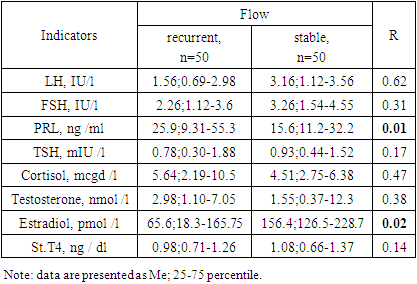
Inactive pituitary adenoma (IPA) or silent pituitary adenoma (Silient Pituitary Adenomas) refers to tumors that secrete one or more hormones or their transcription factors (TFs), detected by immunohistochemical examination (IHC), but this amount of expressed hormones has no clinical significance and is not expressed by symptom complexes [2]. Purpose of the study. To analyze in a comparative aspect the clinical, hormonal and imaging features of inactive pituitary adenomas with a recurrent and stable course. Material and methods. In total, the data of 100 patients with NAH were analyzed: group 1 included 50 patients with a recurrent course, group 2 included 50 patients with a stable course. Results. Both groups were predominantly men under 45 years of age. In the group with a recurrent course, nausea (46% vs. 4.0%; OR 9.80; 95%CI 3.06-31.4; p <0.0001) and dizziness (46.0% vs. 20.0%; OR 3.41; 95% CI 1.40-8.29; p = 0.006) were significantly more common than in the group with a stable course. Along with this, in the group with a recurrent course of the disease, endocrine (100% versus 92.0%) and general somatic (100% versus 76.0%) disorders predominated. A comparative analysis showed that significant factors in the epigenetic modification of the risk of relapse are stress (OR 17.5; 95%CI 6.31-48.4; p<0.0001), brain injury (OR 3.02; 95%CI 1 .27-7.21; p=0.01), obesity (OR 4.89; 95%CI 1.76-13.6; p=0.002) and emotional stress (OR 2.79; 95%CI 1, 11-7.01; p=0.03).
Keywords: Inactive pituitary adenomas, Recurrent course, Stable course
Cite this paper: Kholova Dilorom Sharifovna, Halimova Zamira Yusufovna, Clinical, Hormonal and Imaging Features of Inactive Pituitary Adenomas with Recurrent and Stable Course, American Journal of Medicine and Medical Sciences, Vol. 14 No. 7, 2024, pp. 1862-1868. doi: 10.5923/j.ajmms.20241407.30.
Article Outline
1. Introduction
- Inactive “silent” pituitary adenomas account for about 10–20% of all intracranial tumors and 15–30% of all pituitary adenomas [7]. These tumors are observed in different age groups; the manifestation of the disease, as a rule, occurs in the 4th–8th decade of life [1]. According to literature reviews, there is a statistically slight male predominance in the detection of IPA, although not all studies observed this trend of sexual differentiation [4,6,26]. There are studies showing that men, as a rule, live longer, thereby the risk of developing tumors is greater than that of women [4].Clinical and morphological diagnosis of IPA is difficult, which affects the progression, treatment and prognosis. In most cases, due to the small size and intrasellar location of IPA, incapable of invasive-infiltrative growth, the practical impossibility of their visualization using CT and MRI for tumors up to 1 mm in size, as well as the absence of reliable biochemical tumor markers in the blood, is difficult to reliably assess tactics of therapy, dynamics of the disease, the effectiveness of the treatment [11,18].IPA are mainly characterized as benign tumors and rarely metastasize to nearby structures, however, a significant proportion of tumors demonstrate invasive growth and a recurrent course [23]. The risk of relapse is higher for tumors where there is residual tumor tissue or residual tissue after transsphenoidal adenomectomy (TAG), in cases where there is invasive adenoma growth in the structures of the cavernous sinus [5,11,24].Unlike hormonally active adenomas, IPA does not have a clinical syndrome due to unregulated secretion of hormones, but it should also be noted that the cytoplasm of adenoma cells may contain pituitary hormones and, in this case, they are called silent adenomas - “Silent” adenomas " [2,3]. IPA, compared with functional pituitary adenomas, has unique clinical characteristics in various aspects. First, IPA is usually observed in older age groups compared to hormonally active functional adenomas. Secondly, patients mainly have signs and symptoms of compression or mass effect. Third, a large number of patients have hypopituitarism in one or more adenohypophyseal cell lines. On the other hand, many patients with IPA may not have clinical symptoms for many years and are detected by chance, which leads to difficulties in early diagnosis and compilation of epidemiological characteristics of this disease. The frequency of asymptomatic IPA varies according to the literature from both local and foreign sources. In connection with the above data, clinically non-functioning or inactive pituitary adenomas are adenomas that do not lead to excessive secretion of pituitary hormones leading to corresponding dishormonal clinical syndromes [8].
2. Purpose of the Study
- To analyze in a comparative aspect the clinical, hormonal and imaging features of inactive pituitary adenomas with a recurrent and stable course.
3. Materials and Methods
- In total, the data of 100 patients with NAH were analyzed: group 1 included 50 patients with a recurrent course, group 2 included 50 patients with a stable course.
4. Statistical Analysis
- Statistical processing of the results was carried out using Microsoft programs Excel, IBM SPSS Statistica 23 and MedCalc version 18.5. The initial data were assessed for compliance with normal distribution using the Kolmogorov-Smirnov test. Results are presented as median (Me) [interquartile range Q 25; Q 75], as well as M± SD. Differences were considered statistically significant at p <0.05.
5. Results and Discussions
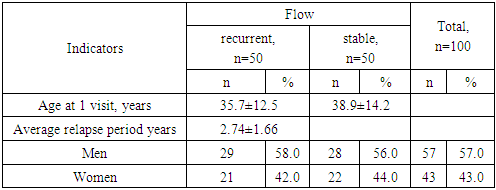
- Data from 100 patients with IPA were analyzed: group 1 included 50 patients with a recurrent course, group 2 included 50 patients with a stable course. By age (group 1 – 35.7±12.5 years; group 2 – 38.9±14.2 years; p=0.24) and gender group (group 1 – men – 58.0% and women – 42.0%; 2 – men – 56.0% and women – 44.0%; OR 1.09; 95% 0.49-2.40) were comparable (Table 1)).
|
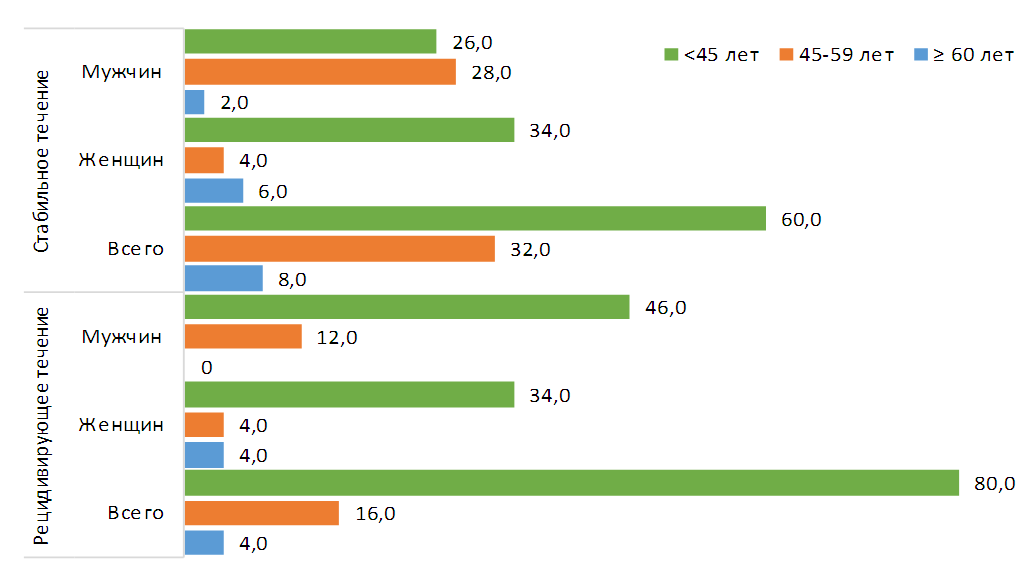 | Figure 1. Gender and age composition of those surveyed |
|
|
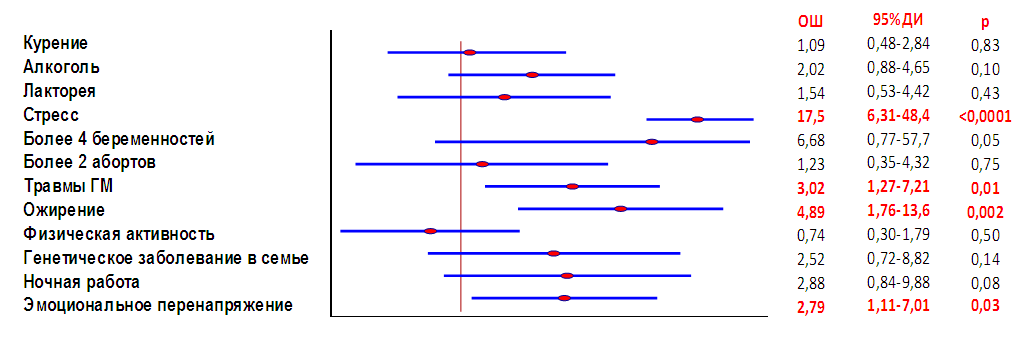 | Figure 2. " Forest " factor diagram epigenetic modification of the risk of IPA relapse |
|
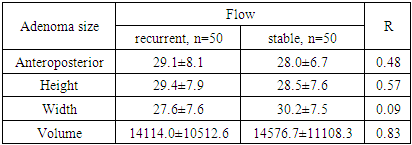
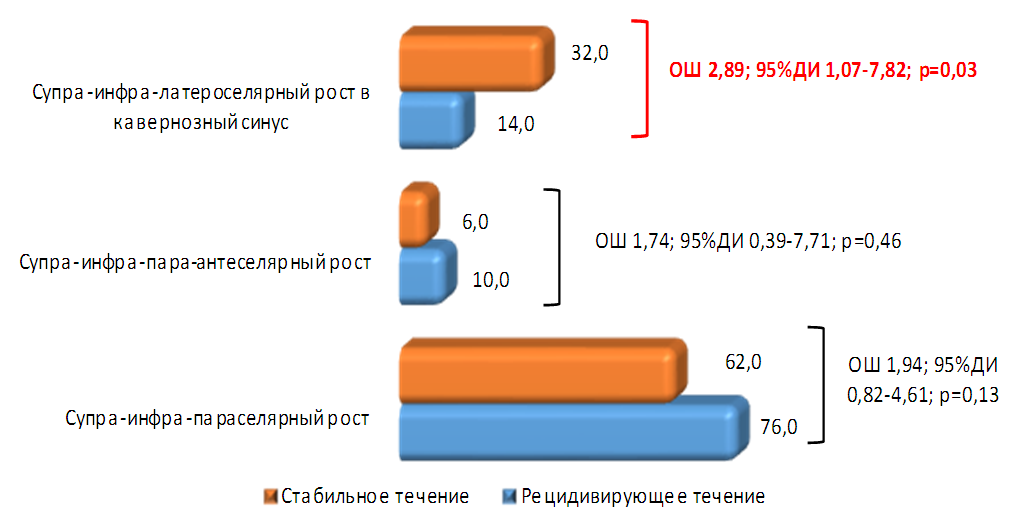 | Figure 3. Direction of tumor growth in patients with NAH |
|
6. Conclusions
- The diagnosis of IPA, as a rule, is established when the tumor reaches a significant size, causing visual impairment, endocrine, neurological, and autonomic disorders associated with compression of the structures of the anterior pituitary gland [3,24]. In this regard, IPA represents one of the difficult problems for neuroendocrinologists, since it is diagnosed late, usually at the stage of symptoms of extrasellar tumor spread [3]. Despite the fact that clinical, hormonal and imaging indicators of inactive pituitary adenomas with recurrent and stable courses had a diverse picture, they did not have comparative features. The study proves that large adenomas exhibit polysymptoms, vivid clinical manifestations of symptoms, and, moreover, have many complications leading to disruption not only of the endocrine system, but also of other organs and systems. The results of these data do not have prognostic significance in relation to indicators of early diagnosis of the course of the disease and in particular the criteria for recurrence and/or stabilization of tumor formation processes, assessing the prediction of treatment outcomes for this group of tumors. And this indicates the importance of using immunohistochemical studies to prevent a recurrent course. The next stage in the development of neuroendocrinology and pituitary surgery should be the introduction of IHC - studies that allow us to determine hormonal, mitotic activity, as well as determine genetic indicators in tumor tissues rather than in the blood, which will allow us to clearly determine the program of measures for patients in the postoperative period and predict their unfavorable outcomes in the form of tumor recurrence, apoplexy, aggressive growth, parasellar neurovascular formations, reducing the incidence of development and often ex juvantibus use of anti-relapse radiation therapy, which contributes to the development of post-radiation encephalopathy, optic nerve atrophy, increased frequency of stroke, panhypopituitarism, chaining these patients for lifelong disability.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML