-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(7): 1859-1861
doi:10.5923/j.ajmms.20241407.29
Received: Jun. 22, 2024; Accepted: Jul. 17, 2024; Published: Jul. 20, 2024

Assessment of Vitreous Spectrum Parameters in Patients with Vitreous Destruction in the Course of Treatment
Azimov S. U.1, Usmanova D. D.2, Kasimova M. S.1
1Center for the Development of Professional Qualifications of Medical Workers, Tashkent, Uzbekistan
2Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Opacities of the vitreous body that occur with age are the result of a violation of the structure of the vitreous body of the processes of its destruction, liquefaction and wrinkling and are currently an urgent problem of ophthalmology that determines the quality of life of patients. According to the literature, about 76% of people have floating flies in front of their eyes, and 33% associate them with a decrease in vision. Vitreous opacities are one of the manifestations of vitreous destruction with the formation of seals that shield light, cast a shadow on the retina, and, as a result, reduce not only the quality of vision of patients, but also their quality of life in general.
Keywords: Ophthalmology, Vitreous, Ultrasound examination
Cite this paper: Azimov S. U., Usmanova D. D., Kasimova M. S., Assessment of Vitreous Spectrum Parameters in Patients with Vitreous Destruction in the Course of Treatment, American Journal of Medicine and Medical Sciences, Vol. 14 No. 7, 2024, pp. 1859-1861. doi: 10.5923/j.ajmms.20241407.29.
1. Introduction
- Under normal conditions, the concentration of cholesterol in the blood significantly exceeds the concentration of cholesterol in the vitreous body. One of the suggested mechanisms of scintillation synchisis pathogenesis is the release of cholesterola from lysed red blood cells [2,5,6,9]. This could explain the cases associated with vitreous hemorrhage, since extravascular blood degeneration releases excess cholesterol, which precipitates as crystals from the vitreous solution.However, this mechanism of cholesterol development due to blood degeneration is not sufficient to explain cases of scinchisis that occur without hemorrhage, such as cases of retinal detachment or overripe cataracts [1,3,4,5]. It is assumed that in retinal detachment, cholesterol is formed from the subretinal fluid that diffuses into the posterior chamber through retinal tears [3,8]. A recent study shows that oxidative stress and lipoperoxidation are the causes of the formation of intraocular cholesterol crystals [4,7,11-0]. This suggests that redox imbalance and increased oxidative modifications in patients with vitreous degradation play a role in pathogenesis, possibly due to changes in the hemato-water or hemato-vitreous barrier [6,7,8]. Treatment of vitreous opacities is currently attracting the attention of doctors and researchers [5,7,8,9,10]. There are many advanced solutions to this problem. In our work, we included the drug atoris in the treatment regimen, and depending on the chosen therapy, we divided all patients into 2 groups.Objective: to determine the level of the lipid spectrum in patients with vitreous destruction.
2. Material and Methods of Research
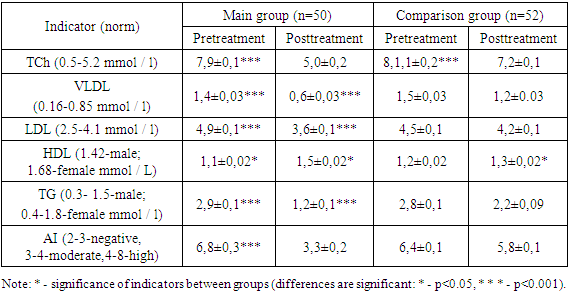
- The study included 102 patients with vitreous destruction. The diagnosis of vitreous destruction was made according to the International Classification of Diseases (ICD-10) - H43. Patients were divided into 2 groups depending on the chosen therapy. Main group 1 consisted of 50 patients with vitreous destruction who received atoris as a background of basic therapy. Comparison group 2 included 52 patients with vitreous destruction who received only basic therapy. Also, to compare laboratory parameters, a control group was created, which included 20 practically healthy individuals , All patients were taken blood to study the parameters of the lipid spectrum. The blood lipid spectrum included: total cholesterol, very low-density lipoproteins (VLDL), low-density lipoproteins (LDL), and high-density lipoproteins (HDL), triglycerides, and the atherogenicity index over the course of treatment.
3. Research Results
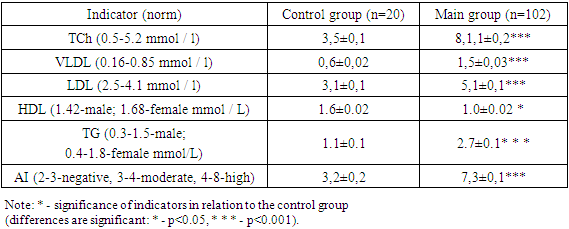
- The results of the study showed (Table 1) thatami revealed a disturbed lipid spectrum in patients with vitreous destruction. When evaluating the analysis of the lipid spectrum in the control group, we found results that did not differ from the reference values: namely, total cholesterol was 3.5±0.1 mmol/L; VLDL-0.6±0.02 mmol/L; LDL-3.1±0.1 mmol/L; triglycerides-1.1±0.1 mmol / L and atherogenicity index – 3.2±0.2; reduced from normal: HDL – 1.6±0.02x mmol/l.In patients of the main group (n=102), total cholesterol was 8.1±0.2 mmol/L; VLDL-1.5±0.03 mmol/L; LDL-5.1±0.1 mmol/L; triglycerides-2.7±0.1 mmol/L and atherogenicity index-7.3±0.1; reduced from the norm: HDL – 1.0±0.02 mmol/l.Thus, analyzing the parameters of the lipid spectrum, we found a deviation from the norm of the indicators of the main group (Table 1).
|
|
4. Conclusions
- The use of the drug atoris in patients of the main group with vitreous destruction compared to the comparison group who did not receive statins, there was a decrease in flies in front of the eyes and floating opacities of the vitreous body.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML