-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(7): 1829-1832
doi:10.5923/j.ajmms.20241407.22
Received: Jun. 3, 2024; Accepted: Jun. 28, 2024; Published: Jul. 19, 2024

Cytomorphological Characteristics of the Respiratory Portion of the Respiratory Tract under the Influence of Chlorpyrifos
Shakhzoda Abdulazizova
Department of Histology and Biology, Fergana Medical Institute of Public Health, Fergana, Uzbekistan
Correspondence to: Shakhzoda Abdulazizova, Department of Histology and Biology, Fergana Medical Institute of Public Health, Fergana, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This article evaluates the cytomorphological effects of chlorpyrifos on the respiratory tract in experimental animals. Materials and Methods: Male and female rabbits (n = 20) were divided into experimental and control groups (by 10 for each group). The experimental group was exposed to aerosolized chlorpyrifos for four months, while the control group was kept under optimal conditions. Lung tissue samples were collected, processed, and examined histologically. Results: Chlorpyrifos exposure resulted in symptoms of poisoning such as rhinorrhea, reduced appetite, and difficulty breathing. Macroscopically, the experimental group's lungs showed edema, discoloration, and firmness, whereas control lungs were healthy. Histological examination revealed expansion of bronchioles, alveolar sac damage, peribronchial infiltrates, thickened alveolar septa with inflammatory cells, and alveolar edema. Conclusion: Chlorpyrifos causes significant pathological changes in lung tissue, indicating a need for careful regulation to prevent health risks.
Keywords: Chlorpyrifos, The respiratory portion of the respiratory tract, Experimental animals, Histological examination, Poisoning, Lung tissue, Pathological changes
Cite this paper: Shakhzoda Abdulazizova, Cytomorphological Characteristics of the Respiratory Portion of the Respiratory Tract under the Influence of Chlorpyrifos, American Journal of Medicine and Medical Sciences, Vol. 14 No. 7, 2024, pp. 1829-1832. doi: 10.5923/j.ajmms.20241407.22.
Article Outline
1. Introduction
- Cases of acute and chronic pesticide intoxication during production processes are becoming increasingly widespread and cause growing concern worldwide [1]. Approximately 3 million cases of acute poisoning and 220,000 fatalities from pesticide exposure are registered annually [2]. The effects of both acute and chronic poisoning include symptoms ranging from mild to severe intoxication, impacting all vital organ systems and exhibiting neurotoxic, carcinogenic, cardiotoxic, nephrotoxic, pulmotoxic, and immunosuppressive effects [3]. Studies have demonstrated that pesticide exposure inhibits surfactant function, while in vivo experiments with mice have shown a consequent reduction in tidal volume [4]. Furthermore, an increased frequency of mitochondrial DNA (mtDNA) somatic mutations has been observed in lung tissues exposed to pesticides. This finding suggests a potential link between pesticide exposure and the genetic integrity of mitochondrial DNA in lung cells, indicating a possible mechanism for the deleterious health effects associated with such environmental toxins [5]. Additionally, Studies have demonstrated that low-dose pesticide exposure induces local B lymphocytes in the lungs to express MHC II molecules, thereby activating T helper cells. This immune response highlights a critical pathway through which pesticides may contribute to pulmonary immune dysfunction [6]. One such pesticide is chlorpyrifos, classified as a moderately hazardous class II insecticide by the WHO [7]. Chlorpyrifos belongs to the large family of organophosphorus compounds. The primary mechanism of action of organophosphorus pesticides is the inhibition of neuronal cholinesterase activity, a key enzyme involved in neurotransmission, leading to the accumulation of acetylcholinesterase in the synaptic cleft [8]. Excessive stimulation of muscarinic and nicotinic acetylcholine receptors in the postsynaptic membrane results in clinical signs and symptoms of organophosphate pesticide poisoning [9]. Cases of laryngospasm, bronchoconstriction, bronchorrhea, and paralysis of respiratory muscles, including the diaphragm, which complicates breathing, have been noted [10]. Thus, the review of scientific literature raises questions regarding cellular changes in the respiratory system, particularly the respiratory portion of the respiratory system, under the influence of chlorpyrifos. This research area is relevant due to the increasing incidence of occupational diseases worldwide. This article evaluates the cytomorphological characteristics of the respiratory portion of the respiratory system under the influence of chlorpyrifos.
2. Material and Methods
- The study was conducted in the Department of Histology and Biology at the Fergana Medical Institute of Public Health, using standard histological methods. Protocol is reviewed and approved by the ethical committee of the Fergana Medical Institute of Public Health according to national and international guidelines such as the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals. The material of research were the New Zealand white rabbit 20 in number and weighing 2.6-2.8 kg, consisting of 10 males and 10 females. In a vivarium, sexually mature animals were housed singly in cages measuring 60x40x35 cm³ to avoid fighting injuries and prevent pregnancy. Cages contain solid floors to prevent foot lesions in rabbits. Animals have been kept at the optimal macroenvironmental temperature, 16-22°C and fed with hay, grass, vegetables, plants, and occasionally fruits as a treat a few times a week. The animals were divided into experimental and control groups, with 10 rabbits in each group, consisting of 5 males and 5 females rabbits. Over four months, the experimental group was exposed to aerosol intoxication with a pesticide containing chlorpyrifos. Chlorpyrifos was diluted with cooled boiled water at a 1:50 ratio, and the mixture was sprayed every three days twice a day. The control group was maintained under optimal conditions. After four months, the animals were euthanized according to Institutional Animal Care and Use Committee (IACUC) Protocols by administering an overdose of a barbiturate, such as sodium pentobarbital, intravenously through an ear vein (30 mg· kg-1 body weight (BW)). This method is quick, effective, and generally accepted as humane. Then by intravenously through an ear vein administered heparin at a dose of 300 international units (IU) per kilogram. After 5 minutes the rabbits were exsanguinated through the carotid artery. Subsequently, lung tissue samples measuring 1-2 cm³ were collected, fixed in a 10% neutral formalin solution, and processed for histological examination. The tissue samples were passed through increasing concentrations of alcohol from 60% to 100% and then embedded in paraffin. Histological sections 7-8 µm thick were prepared using a microtome. The sections were deparaffinized and stained with Hematoxylin-Eosin, Van Gieson, and the PAS reaction method. The histological preparations were examined using an MT 5300L light microscope with a digital camera at magnifications ranging from ×100 to ×400 according to recommendations for morphometric studies.
3. Results
- The results indicated that the data analysis for male and female rabbits was approximately equal, with no significant differences observed. The experimental aerosol intoxication with chlorpyrifos resulted in signs of mild to moderate poisoning in the animals, including rhinorrhea, ptyalism, reduced appetite, weight loss, partial alopecia, difficulty breathing, and lacrimation. In contrast, the control animals displayed normal activity, moderate appetite, and weight gain, and were clinically healthy. Comparative analysis of lung tissue macroscopic specimens revealed edema and discoloration in the experimental group, with shades of gray, brown, and reddish hues. Hemorrhagic areas were also observed. Upon palpation, the lung tissue was noted to be firm. In the control group, macroscopic specimens exhibited a bright pink color characteristic of healthy lungs with the typical lobular structure found in rabbits. Additionally, palpation showed the tissue to be soft without any firm areas (Figures 1 and 2).
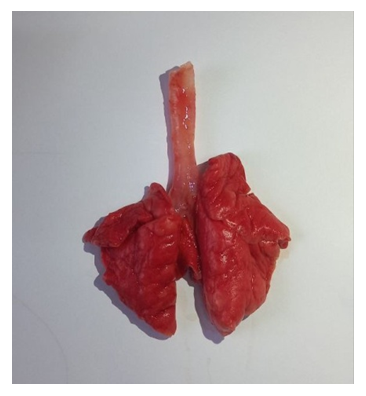 | Figure 1. Macroscopic Specimen of the Lung from a Control Animal |
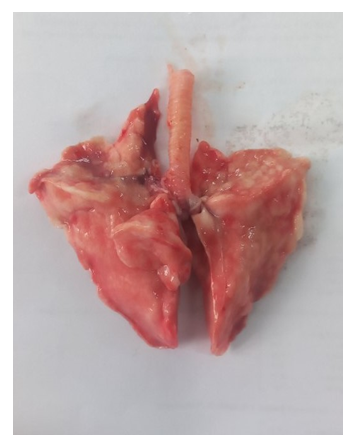 | Figure 2. Macroscopic Specimen of the Lung from an Experimental Animal |
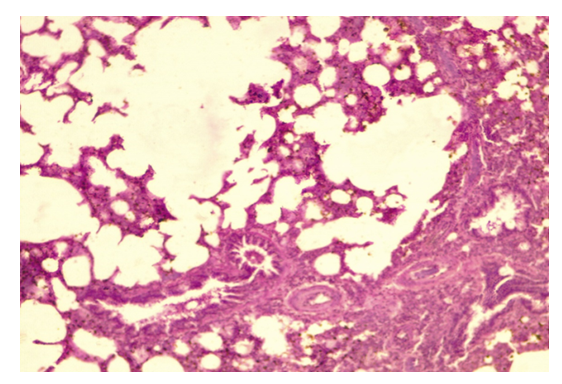 | Figure 3. Lung Parenchyma Showing Emphysematous Bullae, x40, x10 |
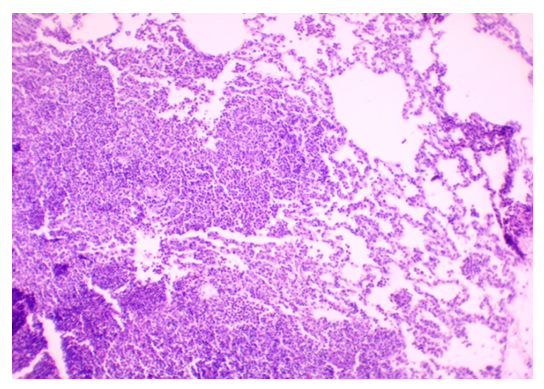 | Figure 4. Lung Parenchyma with Thickened Interalveolar Septa and Lymphocytic Infiltration. Stained with H&E, Obj. x40, Ocular x10 |
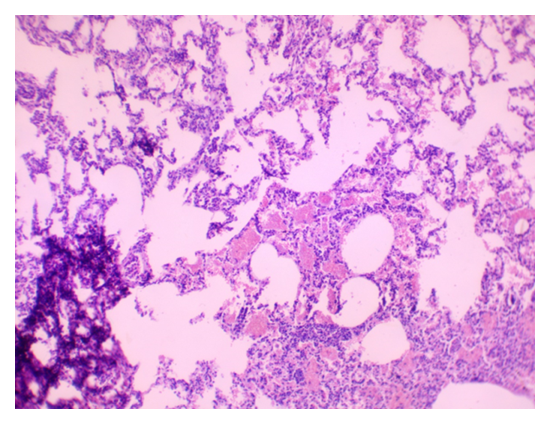 | Figure 5. Lung Parenchyma Showing Thickened Interalveolar Septa and Hemorrhagic Infiltration. Stained with H&E, Obj. x10, Ocular x10 |
4. Discussion
- Our results are consistent with findings from previous researchers studying the effects of chlorpyrofos on the respiratory system. Similar to the study by Akhtar N. and colleagues [11], we found mild toxicity in experimental animals, accompanied by loss of appetite and slight weight loss. Our observations of macroscopic changes in lung tissue are similar to the results of a study by Shaikh N. I. et al. [12], where changes in tissue color and the presence of hemorrhages were also noted. The microscopic changes we found, lymphocytic infiltration, are similar to the findings of a study byYadala, R. et al [13] conducted in Wistar rats.Our observations of septal thickening and emphysematous changes in alveolar tissue are also comparable to the results of the study by Finkelstein, J. N. et al. [14].The study results indicate that experimental aeroallergic intoxication with chlorpyrifos leads to various pathological changes in the lung tissue of animals. Firstly, the expansion of terminal bronchioles and alveolar sacs is observed. This may result from the effects of chlorpyrifos on alveolar epithelial cells and bronchiole walls, reducing the effective area for gas exchange and impairing the breathing process. Secondly, the exfoliation of the terminal epithelium and destruction of the alveolar septa with the formation of bullae or cavities indicate damage to the alveolar tissue structure caused by oxidative stress that led to increased generation of ROS can result in direct oxidant damage and shedding of epithelial cells. This can lead to impaired oxygen and carbon dioxide diffusion through the alveolar-capillary membrane and the development of emphysema. Thirdly, the peribronchial serous infiltrate suggests an inflammatory process around the bronchi, which may result from the action of chlorpyrifos on bronchial structures and lead to bronchitis or bronchopneumonia. The exposure to chlorpyrifos led to an increased expression of TNF-α mRNA, resulting in a synergistic effect. This heightened expression triggers a strong inflammatory response, characterized by significant neutrophil infiltration during lung injury. The thickening of the alveolar septa with neutrophils and macrophages, as well as proteinaceous alveolar edema, may be responses to inflammatory processes and epithelial damage, increasing resistance to gas exchange and worsening tissue oxygenation. Furthermore, the disruption of the integrity of type I pneumocytes and hemorrhagic infiltration can further impair gas exchange and lead to respiratory failure.
5. Conclusions
- Overall, these pathological changes in the lung tissue indicate the severe impact of chlorpyrifos on the respiratory system of animals and underscore the need for control over its use to prevent adverse health effects.
ACKNOWLEDGEMENTS
- The author acknowledges the Department of Histology and Anatomy of Fergana Medical Institute of Public Health, and the Department of Anatomy and Microanatomy of Central Asian Medical Institute.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML