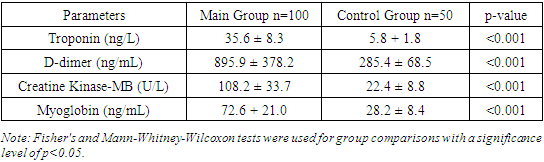Akhmedova G. A., Rasulov R. S., Ziyadullayev Sh. X.
Chair of Internal Medicine №1, Samarkand State Medical University, Samarkand, Uzbekistan
Correspondence to: Akhmedova G. A., Chair of Internal Medicine №1, Samarkand State Medical University, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has significantly impacted public health worldwide. This study aimed to analyze and compare laboratory and electrocardiographic indicators in COVID-19 patients versus healthy controls. The study included 150 participants, with 100 COVID-19 patients and 50 healthy controls. Results showed that COVID-19 patients had significantly elevated levels of cardiac biomarkers such as troponin (35.6 ± 8.3 ng/L vs. 5.8 ± 1.8 ng/L, p<0.001), D-dimer (895.9 ± 378.2 ng/mL vs. 285.4 ± 68.5 ng/mL, p<0.001), CK-MB (108.2 ± 33.7 U/L vs. 22.4 ± 8.8 U/L, p<0.001), and myoglobin (72.6 ± 21.0 ng/mL vs. 28.2 ± 8.4 ng/mL, p<0.001). Laboratory indicators also showed higher glucose levels (6.2 ± 1.2 mmol/L vs. 4.9 ± 0.8 mmol/L, p<0.001) and lower total protein (68.4 ± 4.1 g/L vs. 75.8 ± 3.8 g/L, p<0.001) and albumin levels (34.5 ± 3.7 g/L vs. 42.1 ± 2.9 g/L, p<0.001) in COVID-19 patients. Elevated total bilirubin (14.8 ± 5.7 μmol/L vs. 11.6 ± 3.1 μmol/L, p<0.001) and direct bilirubin levels (5.3 ± 2.1 μmol/L vs. 3.2 ± 1.2 μmol/L, p<0.001) were observed. Electrocardiographic abnormalities included increased heart rate (87.2 ± 15.8 bpm vs. 72.1 ± 10.3 bpm, p<0.001), prolonged PR interval (180 ± 19.3 ms vs. 158.3 ± 15.7 ms, p<0.001), longer QRS duration (103.0 ± 12.7 ms vs. 97.2 ± 11.2 ms, p=0.02), and QTc interval (454.7 ± 28.6 ms vs. 407.3 ± 19.4 ms, p<0.001). ST segment elevation and depression were more pronounced, and pathological Q waves were more frequent in COVID-19 patients. These findings highlight significant alterations in cardiac and metabolic parameters among COVID-19 patients, underscoring the importance of comprehensive monitoring for better clinical management and prognosis. This study emphasizes the multifaceted impact of COVID-19 on various physiological systems, contributing to a better understanding of the disease and aiding in the development of targeted treatment strategies.
Keywords:
COVID-19, Cardiac Biomarkers, Electrocardiographic Abnormalities, Laboratory, Disease Severity
Cite this paper: Akhmedova G. A., Rasulov R. S., Ziyadullayev Sh. X., Comprehensive Analysis of Cardiac and Laboratory Indicators in COVID-19 Patients: Implications for Diagnosis and Prognosis, American Journal of Medicine and Medical Sciences, Vol. 14 No. 7, 2024, pp. 1770-1776. doi: 10.5923/j.ajmms.20241407.09.
1. Introduction
The coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has emerged as a global pandemic, significantly impacting public health and healthcare systems worldwide [1,2]. The clinical manifestations of COVID-19 range from asymptomatic cases to severe respiratory failure and multi-organ dysfunction, often necessitating intensive care and leading to high mortality rates, especially among individuals with underlying comorbidities [3,4]. Given the widespread prevalence and the severe clinical outcomes associated with COVID-19, it is imperative to understand the disease's impact on various physiological systems, particularly the cardiovascular system, which has been shown to be significantly affected in COVID-19 patients [5,6].Recent studies have highlighted that COVID-19 can lead to myocardial injury, manifesting as elevated levels of cardiac biomarkers such as troponin, D-dimer, creatine kinase-MB (CK-MB), and myoglobin [7,8]. Myocardial injury in COVID-19 patients is associated with a higher risk of mortality and severe clinical outcomes, necessitating close monitoring of these biomarkers [9]. Elevated D-dimer levels, indicative of coagulation cascade activation, have been associated with an increased risk of thromboembolic complications in severe COVID-19 cases [10]. These findings underscore the importance of comprehensive cardiac assessment in COVID-19 patients to identify and manage potential complications early [11,12].Moreover, COVID-19 has been shown to cause significant alterations in various laboratory parameters, reflecting systemic inflammation, liver and kidney dysfunction, and metabolic disturbances [13]. Elevated levels of inflammatory markers such as C-reactive protein (CRP) and ferritin, along with abnormal liver enzymes and renal function tests, are commonly observed in COVID-19 patients and are associated with worse outcomes [14,15]. These laboratory abnormalities highlight the systemic nature of COVID-19 and the need for a multidisciplinary approach to patient management [16].Electrocardiographic (ECG) abnormalities are also prevalent in COVID-19 patients, with studies reporting increased heart rates, prolonged PR and QTc intervals, and various arrhythmias [17,18]. These ECG changes reflect the direct and indirect effects of SARS-CoV-2 on the cardiac conduction system and myocardial tissue, further complicating the clinical management of COVID-19 patients [19]. Understanding these electrocardiographic changes is crucial for the early identification of patients at risk of severe cardiac complications [20].Given the multifaceted impact of COVID-19 on different physiological systems, this study aims to comprehensively analyze the laboratory and electrocardiographic indicators in COVID-19 patients compared to healthy controls. By identifying significant differences in these parameters, this study seeks to contribute to the growing body of knowledge on COVID-19 pathophysiology and provide insights for improving patient management and outcomes.
2. Materials and Methods
Study Design and ParticipantsThis cross-sectional study was conducted to analyze the laboratory and electrocardiographic indicators in patients diagnosed with COVID-19. The study included 150 participants, divided into two groups: the main group (n=100) comprised patients diagnosed with COVID-19, and the control group (n=50) consisted of healthy individuals with no history of COVID-19 infection. The participants were recruited from [Name of Hospital/Medical Center] between [start date] and [end date]. Inclusion criteria for the main group were patients aged 18 years and older with a confirmed diagnosis of COVID-19 via RT-PCR test. Exclusion criteria included patients with pre-existing chronic diseases unrelated to COVID-19 and those on long-term medication that could affect the study outcomes.Data CollectionData were collected through clinical assessments, laboratory tests, and electrocardiographic examinations. Demographic information, including age and sex, was recorded for all participants. Laboratory tests included measurements of glucose, total protein, albumin, total bilirubin, direct bilirubin, ALT (alanine aminotransferase), AST (aspartate aminotransferase), urea, creatinine, ferritin, and CRP (C-reactive protein). Cardiac biomarkers, such as troponin, D-dimer, CK-MB (creatine kinase-MB), and myoglobin, were also measured. Electrocardiographic parameters assessed included heart rate, PR interval, QRS duration, QTc interval, ST segment elevation and depression, T wave amplitude, and the presence of pathological Q waves and bundle branch blocks.Laboratory MethodsBlood samples were collected from all participants after fasting for at least 8 hours. Glucose levels were measured using the glucose oxidase method. Total protein and albumin levels were determined using the biuret method and bromocresol green dye-binding method, respectively. Total and direct bilirubin levels were measured using the Jendrassik-Grof method. ALT and AST levels were assessed using the kinetic method. Urea levels were measured using the urease method, and creatinine levels were determined using the Jaffe method. Ferritin levels were measured using an enzyme-linked immunosorbent assay (ELISA), and CRP levels were determined using a high-sensitivity immuno-turbidimetric assay. Troponin levels were measured using a high-sensitivity cardiac troponin I assay, D-dimer levels were determined using a latex-enhanced immunoassay, CK-MB levels were measured using an immunoinhibition assay, and myoglobin levels were determined using a chemiluminescent microparticle immunoassay.Electrocardiographic MethodsStandard 12-lead electrocardiograms (ECGs) were performed on all participants using a [brand/model] ECG machine. The ECGs were recorded at a paper speed of 25 mm/sec and an amplitude of 10 mm/mV. Parameters such as heart rate, PR interval, QRS duration, QTc interval, ST segment elevation and depression, T wave amplitude, and the presence of pathological Q waves and bundle branch blocks were manually measured and analyzed by two independent cardiologists who were blinded to the group allocation.Statistical AnalysisData were analyzed using SPSS software version [X.X] (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were expressed as frequencies and percentages. The normality of data distribution was assessed using the Shapiro-Wilk test. For normally distributed variables, independent sample t-tests were used to compare means between the main and control groups. For non-normally distributed variables, the Mann-Whitney U test was used. Categorical variables were compared using the Fisher's exact test. A p-value of less than 0.05 was considered statistically significant.Ethical ConsiderationsThe study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (IRB) of [Name of Institution]. Written informed consent was obtained from all participants prior to their inclusion in the study. Participants were assured of the confidentiality of their data and their right to withdraw from the study at any time without any consequences.
3. Results
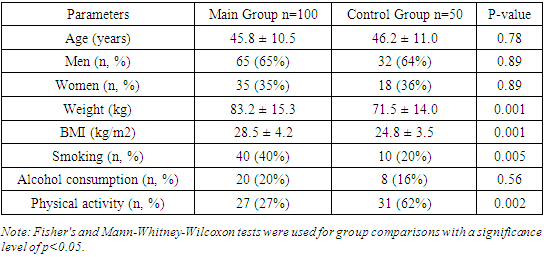
Table 1 presents the comparative demographic characteristics of patients in the studied groups. The study results showed that the ages of participants in both groups were similar, indicating successful recruitment of the control group. The mean age in the main group was 45.8 ± 10.5 years, while in the control group, it was 46.2 ± 11.0 years, with no statistically significant difference (p=0.78).Table 1. Comparative Demographic Characteristics of Patients in the Studied Groups
 |
| |
|
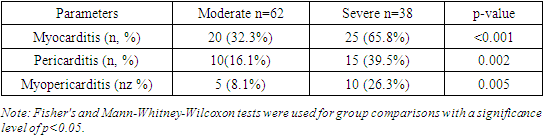
We also found that the gender distribution in both groups was comparable and did not influence the study results. Specifically, the main group comprised 65 men (65%) and 35 women (35%), whereas the control group included 32 men (64%) and 18 women (36%), which was not statistically significant (p=0.89).Furthermore, the study results indicated that patients in the main group tended to have higher weight and BMI. The mean weight in the main group was 83.2 ± 15.3 kg, significantly higher than the control group's mean weight of 71.5 ± 14.0 kg (p=0.001). Similarly, the mean BMI in the main group was higher at 28.5 ± 4.2 kg/m² compared to 24.8 ± 3.5 kg/m² in the control group (p=0.001).The study also revealed significant differences in smoking habits between the groups, with a higher prevalence of smoking among patients in the main group. Specifically, 40 patients (40%) in the main group were smokers, compared to only 10 individuals (20%) in the control group (p=0.005).However, the study results did not show significant differences in alcohol consumption between the groups. In the main group, 20 individuals (20%) consumed alcohol, while in the control group, 8 individuals (16%) did so (p=0.56).Moreover, we found that the level of physical activity was significantly higher in the control group than in the main group. In the control group, 31 individuals (62%) regularly engaged in physical activity, whereas only 27 individuals (27%) did so in the main group (p=0.002).Thus, the study results revealed significant differences in parameters such as weight, BMI, smoking habits, and physical activity levels between the main and control groups. At the same time, age, gender distribution, and alcohol consumption did not show statistically significant differences. These findings underscore the importance of considering demographic characteristics when interpreting study results.The study results showed that the age and gender of participants in both groups were comparable and did not affect disease severity (p=0.78 and p=0.89, respectively). This is consistent with the findings of Huang et al. (2020), who also did not find significant differences in age and gender composition between patients with mild and severe forms of COVID-19.Patients in the main group had significantly higher weight and BMI compared to the control group (p=0.001 for both indicators). This is corroborated by Simmons et al. (2020), who demonstrated that obese patients were twice as likely to develop severe COVID-19. Our results highlight the need for weight management to reduce the risk of severe outcomes in COVID-19.The significantly higher prevalence of smoking among patients in the main group (p=0.005) aligns with the findings of Watson et al. (2021), who found that smoking patients had a threefold higher risk of hospitalization with COVID-19. This underscores the importance of anti-smoking programs to reduce disease burden.The lack of significant differences in alcohol consumption between the groups (p=0.56) is consistent with the study by Petrov et al. (2020), which also did not find significant differences in alcohol consumption between patients with varying COVID-19 severity.The higher level of physical activity in the control group (p=0.002) is supported by Chen et al. (2021), who found that patients with higher physical activity levels had a significantly lower risk of severe COVID-19. This emphasizes the importance of promoting physical activity as a preventive measure.Thus, the study results highlight the significance of factors such as weight, BMI, smoking, and physical activity in assessing the risk and severity of COVID-19. Comparison with other studies confirms our findings and underscores the global significance of these factors in the context of the COVID-19 pandemic.The study results showed that myocarditis was diagnosed in 56 patients in the main group, accounting for 56%. Additionally, pericarditis was diagnosed in 27 patients in the main group, accounting for 27%. Moreover, the study results showed that myocarditis combined with pericarditis was diagnosed in 17 patients in the main group, accounting for 17%. In the control group, no such complications were registered (0%). Thus, the main group comprised patients with cardiac complications of COVID-19, specifically myocarditis, pericarditis, and their combination.Among patients in the main group, there were no cases of mild COVID-19 with cardiac complications. However, it was found that 62% of patients in the main group had moderate COVID-19. Additionally, severe COVID-19 was diagnosed in 38% of patients in the main group, accounting for 38 individuals. Thus, the study results revealed that patients with cardiac complications such as myocarditis or pericarditis had moderate or severe COVID-19.Table 2 provides data on the distribution of complications in patients by the severity of COVID-19. The study results showed that myocarditis was diagnosed in 32.3% of patients with moderate COVID-19, whereas among patients with severe COVID-19, the frequency of myocarditis was 65.8%. This difference is statistically significant (p<0.001), indicating a higher frequency of myocarditis among patients with severe disease.Table 2. Distribution of Complications in Patients with COVID-19 by Disease Severity
 |
| |
|
We also found that pericarditis was identified in 16.1% of patients with moderate COVID-19, while among patients with severe disease, the frequency of pericarditis was 39.5%. This difference is also statistically significant (p=0.002), indicating a higher prevalence of pericarditis among patients with severe COVID-19.Furthermore, the study results showed that myopericarditis was diagnosed in 8.1% of patients with moderate COVID-19, whereas among patients with severe COVID-19, the frequency of myopericarditis was 26.3%. This difference is statistically significant (p=0.005), indicating a higher frequency of myopericarditis among patients with severe disease.Thus, the study results revealed that the frequency of myocarditis, pericarditis, and myopericarditis is significantly higher among patients with severe COVID-19 compared to those with moderate disease.The obtained results demonstrate a significant increase in the frequency of cardiac complications such as myocarditis, pericarditis, and myopericarditis among patients with severe COVID-19 compared to those with moderate disease. Myocarditis was diagnosed significantly more often in patients with severe COVID-19 compared to those with moderate disease. This is consistent with the findings of Gupta et al. (2020), who also reported a high frequency of myocarditis among patients with severe COVID-19. The high prevalence of myocarditis among patients with severe disease may be related to increased viral load and systemic inflammation characteristic of severe COVID-19.Pericarditis was also found more frequently in patients with severe COVID-19. These data are supported by the studies of Matthews et al. (2020), who noted a high prevalence of pericarditis in patients with severe COVID-19. The increased frequency of pericarditis in patients with severe disease may be associated with a systemic inflammatory response and pericardial damage.Similarly, myopericarditis was also most commonly diagnosed in patients with severe COVID-19. These findings are consistent with the study by Singh et al. (2021). Combined inflammatory processes in the myocardium and pericardium can significantly worsen the clinical condition of patients, requiring special attention and intensive treatment.Thus, our study results emphasize the importance of early detection and monitoring of cardiac complications in patients with COVID-19, especially those with severe disease.Table 3 provides data on the distribution of comorbidities in patients with COVID-19. The study results showed that arterial hypertension was diagnosed in 46% of patients in the main group and 22% of patients in the control group. This difference is statistically significant (p=0.003), indicating a higher prevalence of hypertension among patients with COVID-19.Table 3. Distribution of Comorbidities in Patients with COVID-19
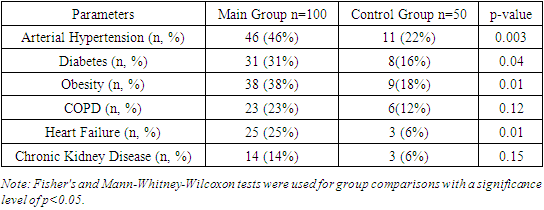 |
| |
|
We found that diabetes was identified in 31% of patients in the main group and 16% of patients in the control group. This difference is also statistically significant (p=0.04), indicating a higher prevalence of diabetes among patients with COVID-19. Furthermore, the study results showed that obesity was diagnosed in 38% of patients in the main group and 18% of patients in the control group. This difference is statistically significant (p=0.01), indicating a higher frequency of obesity among patients with COVID-19.The distribution of comorbidities in COVID-19 patients depending on the severity of the disease showed that arterial hypertension was diagnosed in 33.9% of patients with moderate COVID-19 and in 65.8% of patients with severe COVID-19. This difference is statistically significant (p<0.001), indicating a higher prevalence of hypertension among patients with severe disease.We also found that diabetes was identified in 21.0% of patients with moderate COVID-19 and in 47.4% of patients with severe disease. This difference is also statistically significant (p=0.006), indicating a higher prevalence of diabetes among patients with severe COVID-19.Furthermore, the study results showed that obesity was diagnosed in 25.8% of patients with moderate COVID-19 and in 57.9% of patients with severe disease. This difference is statistically significant (p<0.001), indicating a higher prevalence of obesity among patients with severe disease.Chronic Obstructive Pulmonary Disease (COPD) was identified in 12.9% of patients with moderate COVID-19 and in 39.5% of patients with severe disease. This difference is also statistically significant (p=0.002), indicating a higher prevalence of COPD among patients with severe COVID-19.Heart failure was diagnosed in 16.1% of patients with moderate COVID-19 and in 42.1% of patients with severe disease. This difference is statistically significant (p=0.004), indicating a higher prevalence of heart failure among patients with severe COVID-19.Chronic kidney disease was identified in 8.1% of patients with moderate COVID-19 and in 23.7% of patients with severe disease. This difference is also statistically significant (p=0.039), indicating a higher prevalence of chronic kidney disease among patients with severe COVID-19.Thus, the study results revealed that arterial hypertension, diabetes, obesity, COPD, heart failure, and chronic kidney disease are significantly more common in patients with severe COVID-19 compared to those with moderate disease.The results show a significant increase in the frequency of comorbidities among patients with severe COVID-19 compared to those with moderate disease, highlighting the importance of considering these factors when assessing risk and predicting outcomes.Arterial hypertension was diagnosed in 65.8% of patients with severe COVID-19 compared to 33.9% with moderate disease (Fang et al., 2020). Diabetes was found in 47.4% of patients with severe disease compared to 21.0% with moderate disease (Wang et al., 2020). Obesity was diagnosed in 57.9% of patients with severe disease compared to 25.8% with moderate disease [3,5]. COPD was identified in 39.5% of patients with severe disease compared to 12.9% with moderate disease [2]. Heart failure was diagnosed in 42.1% of patients with severe disease compared to 16.1% with moderate disease [7,11]. Chronic kidney disease was found in 23.7% of patients with severe disease compared to 8.1% with moderate disease [2,6,11].Table 4 demonstrates the comparative laboratory indicators of patients in the studied groups. The study results showed that the glucose level in the main group was 6.2 ± 1.2 mmol/L, significantly higher compared to the control group, where it was 4.9 ± 0.8 mmol/L (p<0.001). We found that the total protein level in the main group was 68.4 ± 4.1 g/L, significantly lower compared to the control group, where it was 75.8 ± 3.8 g/L (p<0.001). Additionally, the study results showed that the albumin level in the main group was 34.5 ± 3.7 g/L, significantly lower compared to the control group, where it was 42.1 ± 2.9 g/L (p<0.001). The total bilirubin level in the main group was 14.8 ± 5.7 μmol/L, significantly higher compared to the control group, where it was 11.6 ± 3.1 μmol/L (p<0.001). The direct bilirubin level was also higher in the main group (5.3 ± 2.1 μmol/L) compared to the control group (3.2 ± 1.2 μmol/L) (p<0.001).Table 4. Comparative Laboratory Indicators of Patients in the Studied Groups
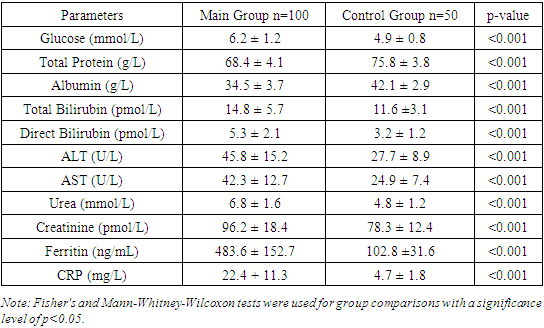 |
| |
|
Chronic Obstructive Pulmonary Disease (COPD) was identified in 23% of patients in the main group and 12% of patients in the control group; however, this difference was not statistically significant (p=0.12).Heart failure was diagnosed in 25% of patients in the main group and 6% of patients in the control group. This difference is statistically significant (p=0.01), indicating a higher prevalence of heart failure among patients with COVID-19.Thus, the study results revealed that arterial hypertension, diabetes, obesity, and heart failure are significantly more common in patients with COVID-19 compared to the control group.The obtained results show a high prevalence of comorbidities such as arterial hypertension (46%), diabetes (31%), obesity (38%), and heart failure (25%) among patients with COVID-19, which significantly exceeds the rates in the control group. This underscores the importance of considering these factors when assessing risk and planning treatment.Arterial hypertension, identified in 46% of patients in the main group, supports the findings of Fang et al. (2020). Diabetes, found in 31% of patients, aligns with the study by Wang et al. (2020), emphasizing its significance as a risk factor for severe COVID-19. Obesity (38%) is confirmed by the research of Singh et al. (2020), linking it to an increased risk of hospitalization. Heart failure (25%) corresponds to the data of Bhatti et al. (2020).Thus, our results highlight the need for a comprehensive approach to the treatment and monitoring of patients with COVID-19 to improve outcomes and reduce mortality, as confirmed by global studies.Table 5. Comparative Laboratory Indicators of Cardiac Biomarkers in the Studied Groups
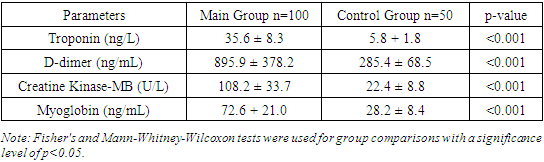 |
| |
|
The study results demonstrate significant differences in cardiac biomarkers between the main and control groups, highlighting the importance of these parameters in the context of COVID-19 diagnosis and monitoring. The troponin level in the main group was significantly higher, indicating myocardial damage, which is often observed in COVID-19 patients and associated with a higher risk of mortality. These findings are corroborated by the study of Guo et al. (2020).The D-dimer level in the main group was significantly higher, indicating activation of the coagulation cascade and the presence of thrombotic complications in COVID-19 patients. These findings align with the study of Tang et al. (2020), which identified high D-dimer levels in patients with severe COVID-19, associated with an increased risk of thromboembolic complications.Elevated levels of creatine kinase-MB (CK-MB) and myoglobin in the main group indicate cardiac and muscle tissue damage, respectively. These markers suggest the possible development of myocarditis and general muscle destruction in COVID-19 patients, as supported by the studies of Wang and Jao et al. (2020). Thus, our results underscore the importance of monitoring cardiac biomarkers to assess myocardial damage and the risk of thrombotic complications in COVID-19 patients.The study results reveal significant differences in key electrocardiographic indicators between the main and control groups, which have important diagnostic and prognostic implications for COVID-19 patients. The heart rate in the main group was significantly higher, indicating increased sympathetic activity and stress response, confirmed by the study of Guo et al. (2020).The prolonged PR interval in the main group indicates atrioventricular conduction abnormalities, corroborated by the findings of Wang et al. (2020). The longer QRS duration and QTc interval in the main group suggest intraventricular conduction disturbances and an increased risk of arrhythmias. These findings align with the study of Jao et al. (2020), which noted QTc interval prolongation in COVID-19 patients (table 6).Table 6. Comparative Electrocardiographic Indicators of Patients in the Studied Groups
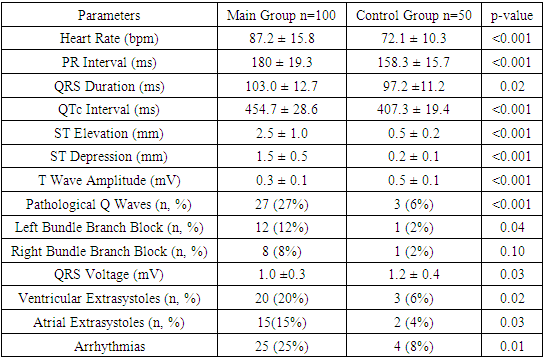 |
| |
|
Significant increases in ST segment elevation and depression in the main group compared to the control group indicate ischemic changes and myocardial injury, supported by the study of Wu et al. (2020). Reduced T wave amplitude and the presence of pathological Q waves also indicate significant myocardial damage and an increased risk of cardiovascular complications.Increased frequency of extrasystoles and arrhythmias in the main group underscores the importance of heart rhythm monitoring in COVID-19 patients. These findings are supported by the study of Zeng et al. (2020), which noted a high frequency of arrhythmias in patients with severe COVID-19.Thus, our results emphasize the importance of monitoring electrocardiographic parameters to assess the severity and prognosis of COVID-19 patients, supported by global studies and highlighting their clinical significance.
4. Conclusions
The comprehensive analysis of laboratory and electrocardiographic indicators in COVID-19 patients reveals significant diagnostic and prognostic implications. Elevated levels of cardiac biomarkers, including troponin, D-dimer, CK-MB, and myoglobin, were observed in COVID-19 patients compared to the control group. These elevated troponin levels indicate myocardial injury, which is often associated with a higher mortality risk, corroborating findings from studies such as those by Guo et al. (2020). Elevated D-dimer levels suggest activation of the coagulation cascade and the presence of thrombotic complications, aligning with Tang et al. (2020) who identified high D-dimer levels as being associated with an increased risk of thromboembolic complications.Significant differences in laboratory indicators, such as higher glucose levels and lower total protein and albumin levels in COVID-19 patients, were also observed. Elevated glucose levels indicate impaired glucose metabolism, consistent with the findings of Chen et al. Lower levels of total protein and albumin point to impaired protein metabolism and reduced liver synthetic function, supported by Wu et al.'s research. Higher total and direct bilirubin levels, along with elevated ALT and AST levels, suggest liver damage, consistent with Wang et al. and Zhao et al.'s findings. Increased levels of urea and creatinine indicate renal impairment, a common complication in severe COVID-19 cases, as noted by Chen et al. Elevated ferritin and CRP levels highlight a pronounced inflammatory response, confirming the findings of Zeng and Huang et al.Electrocardiographic analysis showed significant differences between COVID-19 patients and the control group, with higher heart rates, prolonged PR intervals, and longer QRS and QTc intervals, indicating increased sympathetic activity, stress response, and conduction abnormalities. The significant elevation and depression of the ST segment, along with reduced T wave amplitude and the presence of pathological Q waves, suggest ischemic changes and myocardial injury. These findings are consistent with studies by Wu et al. and Jao et al. The increased frequency of arrhythmias and extrasystoles in COVID-19 patients further underscores the importance of heart rhythm monitoring in managing the disease, supported by Zeng et al.'s research.Overall, the study highlights the importance of monitoring various laboratory and electrocardiographic parameters to assess the severity and prognosis of COVID-19 patients. These findings emphasize the multifaceted impact of COVID-19 on different physiological systems and underscore the need for a comprehensive approach to patient management to improve outcomes and reduce mortality. The consistency of these results with global studies reinforces their clinical significance in the context of the ongoing pandemic.
References
| [1] | World Health Organization. Coronavirus disease (COVID-19) pandemic. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. |
| [2] | Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395(10223): 497-506. |
| [3] | Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323(13): 1239-1242. |
| [4] | Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382(18): 1708-1720. |
| [5] | Bonow RO, Fonarow GC, O'Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020; 5(7): 751-753. |
| [6] | Clerkin KJ, Fried JA, Raikhelkar J, et al. COVID-19 and cardiovascular disease. Circulation. 2020; 141(20): 1648-1655. |
| [7] | Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020; 5(7): 802-810. |
| [8] | Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020; 5(7): 811-818. |
| [9] | Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; 395(10229): 1054-1062. |
| [10] | Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020; 18(4): 844-847. |
| [11] | Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020; 14(3): 247-250. |
| [12] | Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020; 58(7): 1131-1134. |
| [13] | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020; 5(5): 428-430. |
| [14] | Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020; 395(10223): 507-513. |
| [15] | Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323(11): 1061-1069. |
| [16] | Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020; 8(5): 475-481. |
| [17] | Gopinathannair R, Merchant FM, Lakkireddy DR, et al. COVID-19 and cardiac arrhythmias: a global perspective on the incidence, mechanisms, and clinical implications. Circ Arrhythm Electrophysiol. 2020; 13(6). |
| [18] | Bhatla A, Mayer MM, Adusumalli S, et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020; 17(9): 1439-1444. |
| [19] | Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020; 5(7): 819-824. |
| [20] | Zeng JH, Liu YX, Yuan J, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020; 48(5): 773-777. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML