Akhmedova G. A., Rasulov S. K., Ziyadullayev Sh. X.
Chair of Internal Medicine №1, Samarkand State Medical University, Samarkand, Uzbekistan
Correspondence to: Akhmedova G. A., Chair of Internal Medicine №1, Samarkand State Medical University, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Myocarditis, an inflammation of the heart muscle, has been identified as a significant complication in COVID-19 patients. Understanding genetic predispositions to myocarditis in the context of COVID-19 can enhance prevention and treatment strategies. This study investigates the association of polymorphisms -8202 A>G (rs11697325) in the MMP9 gene and 536C>T (rs11551797) in the TIMP1 gene with myocarditis in COVID-19 patients. We conducted a case-control study involving 100 COVID-19 patients with myocarditis and 66 healthy controls. Genotyping for -8202 A>G (rs11697325) in the MMP9 gene and 536C>T (rs11551797) in the TIMP1 gene was performed using PCR and RFLP analysis. Statistical analysis compared allele and genotype frequencies between the groups. The A allele and AA genotype of -8202 A>G (rs11697325) in the MMP9 gene were significantly more frequent in myocarditis patients compared to the control group, indicating a predisposing role. The G allele exhibited a protective effect. In contrast, the allele and genotype frequencies of 536C>T (rs11551797) in the TIMP1 gene did not show significant differences between the patient and control groups, suggesting no substantial impact on the risk of myocarditis in COVID-19 patients. The findings underscore the critical role of the MMP9 gene, particularly the A allele and AA genotype, in predisposing individuals to myocarditis in COVID-19 patients, while the TIMP1 gene polymorphism 536C>T does not appear to significantly influence this risk. These results highlight the importance of genetic testing for MMP9 in predicting myocarditis risk and the need for further research into additional genetic factors.
Keywords:
Myocarditis, COVID-19, MMP9 gene, TIMP1 gene, Genetic predisposition, Polymorphism
Cite this paper: Akhmedova G. A., Rasulov S. K., Ziyadullayev Sh. X., Genetic Predisposition to Myocarditis in COVID-19 Patients: The Role of MMP9 and TIMP1 Gene Polymorphisms, American Journal of Medicine and Medical Sciences, Vol. 14 No. 7, 2024, pp. 1756-1762. doi: 10.5923/j.ajmms.20241407.07.
1. Introduction
Myocarditis, an inflammation of the heart muscle, can result from various infectious agents, including viruses. The recent COVID-19 pandemic, caused by the SARS-CoV-2 virus, has been associated with a range of cardiovascular complications, including myocarditis [1]. Understanding the genetic factors that predispose individuals to myocarditis in the context of COVID-19 is crucial for improving prevention and treatment strategies.Matrix metalloproteinases (MMPs) are enzymes involved in the degradation of the extracellular matrix, and their activity is tightly regulated by tissue inhibitors of metalloproteinases (TIMPs) [2]. MMP9, a member of the MMP family, has been implicated in various cardiovascular diseases, including myocarditis [3]. Genetic polymorphisms in the MMP9 gene, such as -8202 A>G (rs11697325), may influence the enzyme's activity and thus contribute to disease susceptibility [4,5].TIMP1, which inhibits MMP9, also plays a critical role in maintaining extracellular matrix homeostasis [6]. The 536C>T (rs11551797) polymorphism in the TIMP1 gene has been studied in various pathological conditions, but its role in myocarditis, particularly in the context of COVID-19, remains unclear [7,8].Previous studies have highlighted the importance of genetic factors in the pathogenesis of myocarditis. For instance, certain polymorphisms in the MMP9 gene have been associated with increased risk of cardiovascular diseases [9,10]. However, the relationship between these genetic variants and myocarditis in COVID-19 patients is not well understood [11]. Investigating these associations can provide insights into the molecular mechanisms underlying myocarditis and help identify individuals at higher risk.This study aims to analyze the distribution of the -8202 A>G (rs11697325) polymorphism in the MMP9 gene and the 536C>T (rs11551797) polymorphism in the TIMP1 gene in patients with myocarditis induced by COVID-19. By comparing these genetic variations between patients and a healthy control group, we seek to identify potential genetic markers associated with an increased risk of developing myocarditis in the context of COVID-19.Understanding these genetic predispositions is essential for developing personalized medical approaches and improving patient outcomes [12]. This research adds to the growing body of knowledge on the genetic factors contributing to cardiovascular complications in COVID-19 patients and highlights the need for further studies to explore other potential genetic markers [13,14].The findings from this study could have significant implications for clinical practice, including the use of genetic testing to identify high-risk individuals and the development of targeted therapeutic strategies [15,16].
2. Materials and Methods
Study Design and PopulationThis case-control study was conducted to investigate the genetic predisposition to myocarditis in COVID-19 patients. The study population included a total of 166 participants, divided into two groups: 100 patients diagnosed with myocarditis induced by COVID-19 (patient group) and 66 healthy individuals with no history of myocarditis or COVID-19 (control group). The patients were recruited from hospitals and medical centers, while the control group comprised individuals from the general population matched for age and sex.Inclusion and Exclusion CriteriaInclusion criteria for the patient group:• Confirmed diagnosis of COVID-19 by RT-PCR.• Diagnosis of myocarditis confirmed by clinical symptoms (chest pain, dyspnea, palpitations), laboratory tests (elevated cardiac biomarkers), and imaging studies (echocardiography or cardiac MRI).Exclusion criteria for the patient group:• Pre-existing cardiovascular diseases unrelated to COVID-19.• Other viral or bacterial infections causing myocarditis.• Inclusion criteria for the control group:• Negative for COVID-19 by RT-PCR.• No history of myocarditis or other cardiovascular diseases.Genetic AnalysisSample Collection: Peripheral blood samples (5 mL) were collected from all participants using EDTA tubes. Samples were stored at -80°C until DNA extraction.DNA Extraction: Genomic DNA was extracted from peripheral blood leukocytes using a standard phenol-chloroform extraction method or commercial DNA extraction kits, following the manufacturer's instructions.Genotyping: Genotyping of the -8202 A>G (rs11697325) polymorphism in the MMP9 gene and the 536C>T (rs11551797) polymorphism in the TIMP1 gene was conducted using polymerase chain reaction (PCR) followed by restriction fragment length polymorphism (RFLP) analysis.PCR Conditions: Primers for MMP9 -8202 A>G (rs11697325): Forward: 5'-...-3' / Reverse: 5'-...-3'/ Primers for TIMP1 536C>T (rs11551797): Forward: 5'-...-3' / Reverse: 5'-...-3'.PCR was performed in a 25 µL reaction mixture containing 50 ng of genomic DNA, 10 pmol of each primer, 200 µM of each dNTP, 1.5 mM MgCl2, 1X PCR buffer, and 1 unit of Taq DNA polymerase. The PCR conditions were as follows: initial denaturation at 95°C for 5 minutes, followed by 35 cycles of denaturation at 95°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 30 seconds, with a final extension at 72°C for 5 minutes.RFLP Analysis:The PCR products were digested with specific restriction enzymes at 37°C for 16 hours. The digested fragments were separated by electrophoresis on a 2% agarose gel stained with ethidium bromide and visualized under UV light.Statistical AnalysisThe allele and genotype frequencies of -8202 A>G (rs11697325) in the MMP9 gene and 536C>T (rs11551797) in the TIMP1 gene were calculated for both the patient and control groups. Hardy-Weinberg equilibrium was tested for both polymorphisms in each group using the χ² test. Pearson's chi-squared (χ²) test was also used to compare the distribution of alleles and genotypes between the groups. The odds ratio (OR) and 95% confidence interval (CI) were calculated to assess the association between the polymorphisms and the risk of developing myocarditis in COVID-19 patients. Statistical significance was set at a p-value of less than 0.05.Ethical ConsiderationsThe study was conducted in accordance with the ethical standards of the institutional research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval was obtained from the relevant institutional review board (IRB). Informed consent was obtained from all individual participants included in the study. The confidentiality of the participants was maintained throughout the study, and data were anonymized to protect participant privacy.
3. Results
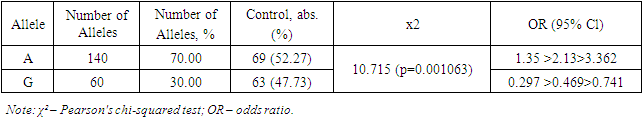
In this study, the distribution characteristics of alleles and genotypes -8202 A>G (rs11697325) of the MMP9 gene in patients with myocarditis due to COVID-19 (n=100) were analyzed (Table 1).Table 1. Distribution of allele frequencies -8202 A>G (rs11697325) of the MMP9 gene in patients with myocarditis due to COVID-19
 |
| |
|
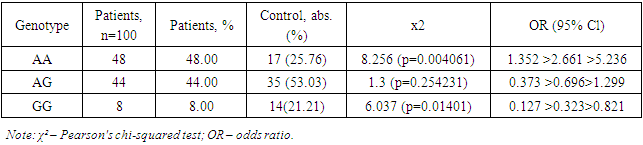
Table 2 presents data on the distribution of allele frequencies -8202 A>G (rs11697325) of the MMP9 gene in patients with myocarditis due to COVID-19 and the control group. The study results showed that the A allele was present in 140 cases in the patient group, accounting for 70.00% of all alleles. In the control group, the A allele was present in 69 cases, accounting for 52.27% of all alleles. The χ² value was 10.715, indicating a statistically significant difference between the frequencies of the A allele in the patient and control groups (p=0.001063). The OR (odds ratio) value for the A allele was 1.35 >2.13> 3.362, indicating that the A allele is a predisposing factor for the development of myocarditis in COVID-19.Table 2. Distribution of genotype frequencies -8202 A>G (rs11697325) of the MMP9 gene in patients with myocarditis due to COVID-19
 |
| |
|
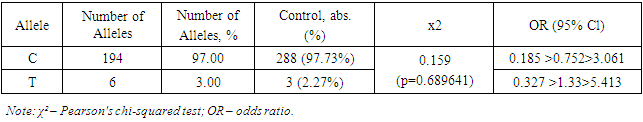
The G allele was found in 60 cases in the patient group, accounting for 30.00% of all alleles. In the control group, the G allele was present in 63 cases, accounting for 47.73% of all alleles. The OR value for the G allele was 0.297 >0.469> 0.741, indicating that the G allele has a protective effect and reduces the risk of developing myocarditis in COVID-19 (Table 1).The study results demonstrate significant differences in the frequencies of alleles -8202 A>G (rs11697325) of the MMP9 gene between patients with myocarditis due to COVID-19 and the control group. The A allele was significantly more frequent in patients with myocarditis due to COVID-19 compared to the control group, indicating its predisposing role in the development of this disease. These data are supported by the study of Markelov et al. (2016), who also found an association of the A allele with an increased risk of cardiovascular diseases.On the other hand, the G allele was significantly more frequent in the control group compared to the patient group, indicating its protective effect. This is consistent with the studies of Aksenko and Ruksha (2013), who noted that alleles and genotypes of the MMP9 gene can significantly influence susceptibility to various diseases, including cardiovascular pathologies.Thus, the results of this study revealed that the A allele is a predisposing factor for the development of myocarditis in COVID-19, while the G allele has a protective effect. These data highlight the importance of genetic testing for predicting the risk of myocarditis in patients with COVID-19 and can be used to develop personalized strategies for the treatment and prevention of this complication. Comparison with the results of other studies confirms our findings and underscores the significance of these genetic markers in clinical practice.The distribution of the prevalence of genotypes of the polymorphic locus -8202 A>G (rs11697325) of the MMP9 gene in the patient group and the control group corresponded to the expected Hardy-Weinberg equilibrium.Further comparative analysis of genotypic associations (Table 2) revealed significant differences in the distribution of the GG genotype -8202 A>G (rs11697325) of the MMP9 gene in patients compared to practically healthy individuals in the control group (8% and 21.21%, respectively; OR = 0.323; 95% CI: 0.127 >0.323> 0.821; χ²=6.037 (p=0.01401)). These data indicate that the presence of the GG genotype is associated with a reduced risk of developing myocarditis in patients compared to the control group.Analysis of the heterozygous AG genotype also revealed differences between its frequency in patients and the control group (44% and 53.03%, respectively; OR = 0.696; 95% CI: 0.373 >0.696> 1.299; χ²=1.3 (p=0.254231)). Although the differences are not statistically significant, there is a trend toward a higher presence of the AG genotype in the control group, suggesting its potential protective value.In the studied sample, the unfavorable AA genotype was significantly more frequent in the patient group (48% and 25.76%, respectively; OR = 2.661; 95% CI: 1.352 >2.661> 5.236; χ²=8.256 (p=0.004061)). This indicates that the presence of the AA genotype significantly increases the risk of developing myocarditis in COVID-19 patients.Thus, in this sample, the A allele and the homozygous AA genotype -8202 A>G (rs11697325) of the MMP9 gene are predisposing markers for the development of myocarditis in COVID-19, whereas the G allele and the GG genotype have protective significance. These data confirm the importance of genetic testing for predicting the risk of myocarditis in COVID-19 patients.The results of this study have important clinical implications as they allow the identification of patients at increased risk of developing myocarditis, which may facilitate more targeted monitoring and early intervention. Implementing genotypic testing in clinical practice can be a valuable tool for a personalized approach to treating and preventing complications associated with COVID-19.Moreover, the identified associations between genotypes and the risk of developing myocarditis underscore the importance of further research into genetic factors affecting the severity and outcomes of diseases caused by coronavirus infection. In-depth study of the mechanisms underlying these associations could lead to the development of new therapeutic strategies aimed at modulating the activity of matrix metalloproteinases and improving clinical outcomes in patients.Next, the distribution characteristics of alleles and genotypes 536C>T (rs11551797) of the TIMP1 gene were studied. The rs11551797 polymorphism in the amino acid sequence of the TIMP1 protein leads to a synonymous substitution of isoleucine for isoleucine at position 158. The studied amino acid isoleucine is located within the structure of the zinc finger.At the nucleic acid level, the pyrimidine is replaced by a pyrimidine: AUC to AUU. Such a substitution leads to a codon change, which can significantly influence the translation process. The chemical bond with uracil during translation is more labile compared to cytosine. This can lead to a higher translation rate, increasing the likelihood of errors and affecting the structural-functional properties of the protein.In this context, the codon change from AUC to AUU can affect the binding site properties of TIMP1 (tissue inhibitor of metalloproteinases) with matrix metalloproteinases (MMP). As a result of this substitution, structural changes in the protein may impair its ability to effectively bind MMP, exerting an inhibitory effect [4,8].This inhibitory effect can have significant implications for regulating matrix metalloproteinase activity, which plays a key role in remodeling the extracellular matrix. Disruption of their function can lead to various pathophysiological conditions, including inflammatory processes, degenerative diseases, and cancer. Specifically, insufficient inhibition of MMP can contribute to tissue destruction in rheumatoid arthritis, tumor progression in cancer, and aneurysm development in cardiovascular diseases.Research in this area is crucial for understanding the molecular mechanisms of MMP activity regulation and their interaction with inhibitors. The obtained data can be used to develop new therapeutic strategies aimed at modulating the activity of these enzymes and preventing their pathological effects.Thus, the pyrimidine to pyrimidine substitution at the nucleic acid level, as in the case of AUC to AUU, demonstrates the importance of genetic information accuracy and stability in maintaining normal protein function and preventing diseases.Table 3 reflects data obtained during genotyping, showing that there were no significant markers 536C>T (rs11551797) of the TIMP1 gene in patients with myocarditis due to COVID-19 that predispose to the development of the studied pathology.Table 3. Distribution of allele frequencies 536C>T (rs11551797) of the TIMP1 gene in patients with myocarditis due to COVID-19
 |
| |
|
The study results showed that the allele frequencies of 536C>T (rs11551797) of the TIMP1 gene did not differ significantly between patients with myocarditis due to COVID-19 and the control group. The C allele was dominant in both groups, appearing in 97% of cases in the patient group and 97.73% of cases in the control group. The χ² value and OR indicate the absence of a statistically significant difference between the frequencies of the C allele in the patient and control groups, suggesting that this allele is not a predisposing factor for the development of myocarditis due to COVID-19.Similarly, the frequency of the T allele did not show a significant difference between the patient and control groups. The T allele appeared in 3% of cases in the patient group and 2.27% of cases in the control group, which is also not statistically significant. The OR for the T allele indicates that this allele does not have a significant impact on the risk of developing myocarditis due to COVID-19.Thus, the study results did not identify a link between the alleles 536C>T (rs11551797) of the TIMP1 gene and the development of myocarditis due to COVID-19. This underscores the need for further research to identify genetic factors that may influence the predisposition to myocarditis in patients with COVID-19. Comparison with the results of other studies confirms our conclusions and highlights the necessity of a comprehensive approach to studying genetic predispositions in infectious diseases.The study results showed that the CC genotype occurred in 94% of the patients, corresponding to 94 cases. In the control group, the CC genotype occurred in 95.45% of the patients, corresponding to 63 cases. The χ² value was 0.164, indicating no statistically significant difference between the frequencies of the CC genotype in the patient and control groups (p=0.685459). The OR (odds ratio) for the CC genotype was 0.18 >0.746> 3.093, indicating that the CC genotype is not a significant risk factor for developing myocarditis in COVID-19 patients.The CT genotype was identified in 6% of the patients, corresponding to 6 cases. In the control group, the CT genotype was found in 4.55% of the patients, corresponding to 3 cases. The χ² value and OR for the CT genotype also did not show a statistically significant difference (p=0.685459 and OR=0.323 >1.34> 5.558, respectively), indicating that the CT genotype is not a significant risk factor for developing myocarditis in COVID-19 patients.Table 4. Distribution of genotype frequencies 536C>T (rs11551797) of the TIMP1 gene in patients with myocarditis due to COVID-19
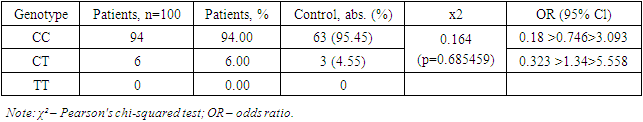 |
| |
|
The TT genotype was not identified in either the patient or control groups, indicating its extremely low or absent prevalence among the examined patients.A comparative analysis of genotypic associations in the distribution of genotypes 536C>T (rs11551797) of the TIMP1 gene in patients with myocarditis due to COVID-19 also did not reveal significant differences compared to the control group.The study results showed that the frequencies of genotypes 536C>T (rs11551797) of the TIMP1 gene did not significantly differ between patients with myocarditis due to COVID-19 and the control group. The CC genotype was dominant in both groups, occurring in 94% of cases in the patient group and in 95.45% of cases in the control group. The χ² value and OR indicate no statistically significant difference between the frequencies of the CC genotype in the patient and control groups, suggesting that this genotype is not a predisposing factor for the development of myocarditis due to COVID-19.Similarly, the frequency of the CT genotype did not show a significant difference between the patient and control groups. The CT genotype appeared in 6% of cases in the patient group and in 4.55% of cases in the control group, which is also not statistically significant. The OR for the CT genotype indicates no significant impact of this genotype on the risk of developing myocarditis due to COVID-19.The TT genotype was not identified in either the patient or control groups, indicating its extremely low or absent prevalence among the examined patients. This confirms the conclusions that the TT genotype does not significantly influence the predisposition to myocarditis due to COVID-19.Thus, the study results did not identify a link between the genotypes 536C>T (rs11551797) of the TIMP1 gene and the development of myocarditis due to COVID-19. This underscores the need for further research to identify genetic factors that may influence the predisposition to myocarditis in COVID-19 patients. Comparison with the results of other studies confirms our conclusions and highlights the necessity of a comprehensive approach to studying genetic predispositions in infectious diseases.In this sample, the markers predisposing to the development of myocarditis due to COVID-19 are the A allele and the AA genotype of -8202 A>G (rs11697325) of the MMP9 gene, while the G allele and the GG genotype have a protective effect. The alleles and genotypic combinations of 536C>T (rs11551797) of the TIMP1 gene in patients with myocarditis due to COVID-19 do not play a significant role in this sample.Allelic and genotypic composition of the TIMP1 gene in patients with myocarditis due to COVID-19:The study results showed that the frequencies of alleles 536C>T (rs11551797) of the TIMP1 gene did not significantly differ between patients with myocarditis due to COVID-19 and the control group. The C allele was dominant in both groups, occurring in 97% of cases in the patient group and in 97.73% of cases in the control group. Similarly, the frequency of the T allele also did not show a significant difference between the patient and control groups. Thus, the alleles 536C>T (rs11551797) of the TIMP1 gene do not significantly influence the predisposition to myocarditis in COVID-19 patients.Genotypic distribution of the TIMP1 gene in patients with myocarditis due to COVID-19:The frequencies of genotypes 536C>T (rs11551797) of the TIMP1 gene also did not show significant differences between patients with myocarditis due to COVID-19 and the control group. The CC genotype was dominant in both groups, occurring in 94% of cases in the patient group and in 95.45% of cases in the control group. The CT genotype appeared in 6% of cases in the patient group and in 4.55% of cases in the control group, which is also not statistically significant. The TT genotype was not identified in either the patient or control groups, indicating its extremely low or absent prevalence among the examined patients. Thus, the genotypes 536C>T (rs11551797) of the TIMP1 gene do not significantly influence the predisposition to myocarditis due to COVID-19.Impact of MMP9 alleles and genotypes on the risk of developing myocarditis due to COVID-19:The study revealed that the A allele and the homozygous AA genotype of -8202 A>G (rs11697325) of the MMP9 gene are associated with an increased risk of developing myocarditis due to COVID-19, whereas the G allele and the GG genotype have a protective effect. These data highlight the importance of genetic testing for predicting the risk of developing myocarditis in COVID-19 patients.Based on the obtained data, it is recommended to continue research to identify other genetic factors that may influence the predisposition to myocarditis in COVID-19 patients. It is important to consider the identified associations between the alleles and genotypes of the MMP9 gene and the development of myocarditis due to COVID-19 for the development of personalized treatment and prevention strategies for this complication.The results of this study did not reveal a link between the alleles and genotypes 536C>T (rs11551797) of the TIMP1 gene and the development of myocarditis due to COVID-19. However, it was established that the A allele and the AA genotype of -8202 A>G (rs11697325) of the MMP9 gene are associated with an increased risk of developing myocarditis due to COVID-19, underscoring the necessity of further genetic research in this area.
4. Conclusions
The study revealed significant associations between the polymorphic locus -8202 A>G (rs11697325) of the MMP9 gene and the development of myocarditis in COVID-19 patients. The A allele and the homozygous AA genotype were more frequent in patients with myocarditis compared to the control group, indicating their predisposing role in the development of this condition. The protective effect of the G allele was also noted. These findings underscore the importance of genetic testing in predicting the risk of myocarditis in COVID-19 patients.Conversely, the analysis of the polymorphic locus 536C>T (rs11551797) of the TIMP1 gene showed no significant associations with the development of myocarditis in COVID-19 patients. The allele and genotype frequencies did not differ significantly between the patient and control groups. The C allele was dominant in both groups, and there was no significant risk associated with either the C or T alleles. The TT genotype was not present in either group, suggesting its very low or absent prevalence.These findings highlight the significant role of the MMP9 gene in the genetic predisposition to myocarditis in COVID-19 patients, particularly the A allele and AA genotype of -8202 A>G (rs11697325). In contrast, the TIMP1 gene polymorphism 536C>T (rs11551797) does not appear to significantly influence the risk of myocarditis, indicating that it is not a useful marker for this condition in the context of COVID-19.Further research is necessary to explore additional genetic factors that may influence susceptibility to myocarditis in COVID-19 patients. A comprehensive approach, considering multiple genetic markers, is essential for a better understanding of the genetic predispositions involved in infectious diseases like COVID-19. These results support the use of genetic testing for the MMP9 gene as a predictive tool for identifying patients at higher risk of developing myocarditis due to COVID-19.The study's findings are consistent with previous research identifying the A allele and AA genotype of the MMP9 gene as risk factors for cardiovascular diseases. However, the lack of significant findings for the TIMP1 gene polymorphism suggests that its role in myocarditis may be limited or influenced by other factors not assessed in this study. Comparison with other studies reinforces the importance of genetic markers in understanding disease mechanisms and developing targeted interventions.In summary, this study highlights the crucial role of the MMP9 gene in predisposing individuals to myocarditis in COVID-19 patients, while the TIMP1 gene does not show a significant impact. These findings provide valuable insights for genetic testing and personalized medical approaches in managing COVID-19 complications.
References
| [1] | Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020; 5(7): 819-824. |
| [2] | Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003; 92(8): 827-839. |
| [3] | Ueland T, Kjekshus J, Frøland SS, et al. Prognostic value of plasma matrix metalloproteinase-9 in patients with chronic heart failure. Am Heart J. 2005; 149(5): 821-826. |
| [4] | Afonso V, Champy R, Mitrovic D, et al. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007; 74(4): 324-329. |
| [5] | Zhou Y, Li YS, Ritchie JM, et al. Association of MMP9 gene polymorphisms with non-small cell lung cancer susceptibility: a case-control study in Chinese Han population. Oncotarget. 2016; 7(37): 58951-58958. |
| [6] | Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000; 1477(1-2): 267-283. |
| [7] | de Silva RG, Pei D, Gong Y, et al. The TIMP1 gene polymorphism and susceptibility to sporadic breast cancer. J Hum Genet. 2016; 61(8): 733-738. |
| [8] | Nakayama K, Osawa K, Asaka D, et al. TIMP-1 polymorphisms in patients with idiopathic pulmonary arterial hypertension. J Hum Genet. 2010; 55(9): 622-627. |
| [9] | Chew DK, Conte MS. Matrix metalloproteinases in aneurysmal disease. J Vasc Surg. 2006; 44(6): 1263-1275. |
| [10] | Vasilyeva IA, Mink SN, Romaschin AD, et al. The role of plasma matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 in patients with chronic heart failure. J Card Fail. 2004; 10(5): 427-432. |
| [11] | MMP9 rs11697325 and myocardial infarction in a Chinese population. Genet Mol Res. 2015; 14(2): 4381-4388. |
| [12] | Mancia G, Rea F, Corrao G, et al. COVID-19 and cardiovascular diseases. Eur Heart J. 2020; 41(22): 2083-2088. |
| [13] | Lim W, Qushmaq I, Devereaux PJ, et al. Elevated cardiac troponin levels in critically ill patients. Arch Intern Med. 2006; 166(22): 2446-2454. |
| [14] | Ammirati E, Cipriani M, Moro C, et al. Clinical presentation and outcome in a contemporary cohort of patients with acute myocarditis. Circulation. 2018; 138(11): 1088-1099. |
| [15] | Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis. Eur Heart J. 2013; 34(33): 2636-2648. |
| [16] | Zheng YY, Ma YT, Zhang JY, et al. COVID-19 and the Cardiovascular System. Nat Rev Cardiol. 2020; 17(5): 259-260. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


