Zulfiya R. Khayruddinova1, Gavhar S. Khaydarova2, Holida E. Shaykhova3, Nozim O. Akhundjanov4, Ranokhon A. Jalolova5
1Tashkent Medical Academy, Researcher, Tashkent, Uzbekistan
2Tashkent Medical Academy, Doctor of Medical Sciences (DSc), Tashkent, Uzbekistan
3Tashkent Medical Academy, Professor, Tashkent, Uzbekistan
4Tashkent Medical Academy, Senior Lecturer, Doctor of Philosophy (PhD), Tashkent, Uzbekistan
5Tashkent Medical Academy, Tashkent, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Gastroesophageal reflux disease occurs in 18-28% of the US population. Up to 10% of the population experiences symptoms of gastroesophageal reflux disease daily, 30% weekly, and 50% of the adult population monthly. Chronic pharyngitis characterized by such symptoms as hoarseness, dysphonia, pain and/or burning in the throat, mucus flow down the nasopharynx, chronic cough, the sensation of a lump in the throat, laryngospasm and dysphagia. Chronic pharyngitis is accompanied by a change in upper gastrointestinal tract confirmed by EGDFS. The examination of esophagus and stomach can be valuable in patients suffering of CP. Our investigation shows the prevalence of catarrhal esophagitis of the lower third of the esophagus on EGDFS which was diagnosed on 46,7% of cases. There are alterations in microbiota of the oropharynx with a predominance of opportunistic microorganisms in quantities exceeding the norm. The use of oral probiotic Streptococcus salivarius K12 in the treatment of chronic pharyngitis seems promising.
Keywords:
Pharynx microbiota, Chronic pharyngitis, Gastroesophageal reflux disease, Extraesophageal syndrome, Oral probiotic
Cite this paper: Zulfiya R. Khayruddinova, Gavhar S. Khaydarova, Holida E. Shaykhova, Nozim O. Akhundjanov, Ranokhon A. Jalolova, Evaluation of the State of Upper Gastrointestinal Tract and Throat Microbiota in Patients with Chronic Pharyngitis, American Journal of Medicine and Medical Sciences, Vol. 14 No. 7, 2024, pp. 1749-1755. doi: 10.5923/j.ajmms.20241407.06.
1. Introduction
According to current epidemiological data, gastroesophageal reflux disease (GERD) occurs in 18-28% of the US population. Up to 10% of the population experiences symptoms of GERD daily, 30% weekly, and 50% of the adult population monthly. In the United States, 44 million people have symptoms of GERD [1,17].About 10% of people in South America, 11.9% in Turkey, and approximately the same number of patients in Europe report symptoms of GERD [17].According to the World Gastroenterological Association, GERD is a sensorimotor disorder associated with disruption of normal antireflux mechanisms (e.g, function of the lower esophageal sphincter, phrenicoesophageal ligament), with changes in normal physiology (e.g, impaired esophageal motility, increased intragastric pressure, increased abdominothoracic pressure gradient), or very rarely, with excessive secretion of gastric juice (Zollinger-Ellison syndrome).Maev I.V. and others studied the otorhinolaryngological status of 78 outpatients suffering from GERD. It was found that chronic pharyngitis was predominantly diagnosed in 55 (70.5%) cases, while only 9 (11.5%) patients had no pathology. The following diseases were also identified: chronic tonsillitis (in 11 patients – 14,5%), chronic laryngitis (in 9 – 11,5%), chronic rhinitis (in 11 people – 14%), chronic sinusitis (in 6 – 7,7%), eustachiitis (in 5 – 6,4%) and chronic adhesive otitis (in 6 patients – 7,7%) [18].According to current researches chronic pharyngitis was found at about 70.5% of patients suffering from GERD. We can suppose a frequent connection between GERD and chronic otorhinolaryngological diseases such as tonsillitis, laryngitis, rhinitis, sinusitis, tubo-otitis and adhesive otitis media diagnosed with GERD (up to 88.5% of cases).Chronic pharyngitis is a chronic inflammation of the pharyngeal mucosa, submucosal layer and lymphoid tissue of the pharynx. Chronic pharyngitis comes out with such symptoms as hoarseness, dysphonia, pain and/or burning in the throat, mucus flow down the nasopharynx, chronic cough, the sensation of a lump in the throat, laryngospasm and dysphagia. Li Z. et others show that the incidence of chronic pharyngitis is about 30% in Chinese population [2,17]. Also, the outpatient visits for chronic pharyngitis in the US range from 7 million to 11 million per year [1,17].Shen Y et al. found the increasement of frequency of chronic pharyngitis (CP) for 10-20% of pharyngeal diseases and 12-14% of all otorhinolaryngological diseases [23].The main causes of chronic pharyngitis can be divided into two groups: non-infectious and infectious. We will not discuss infectious causes of chronic pharyngitis in this article, and will focus on non-infectious factors. The last are as follows: smoking, mechanical traumas, chronic rhinitis, medications (inhaled glucocorticosteroids, ACE inhibitors, chemical preparations, radiation therapy), inhalation of chemicals (ozone, oxides of sulfur, nitrogen, boron, cement dust, gasoline, etc.), concomitant diseases (thyroiditis, Kawasaki desease, etc.), GERD [18].Manifestations of GERD are divided into esophageal and extraesophageal syndromes (see Fig. 1). Among extraesophageal diseases, there are reliably associated diseases - reflux cough, reflux laryngitis, reflux asthma, reflux erosive lesions of teeth, and unreliably associated diseases - pharyngitis, sinusitis, idiopathic pulmonary fibrosis, recurrent otitis media [24].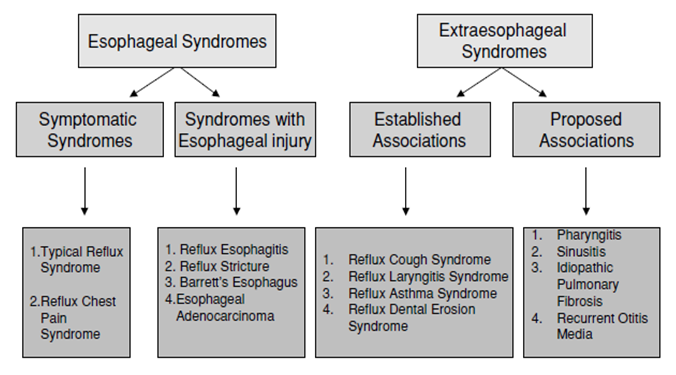 | Figure 1. The overall definition of GERD and its constituent syndromes |
The oral cavity is truly considered the second largest microbiota in the body after the intestinal cavity. About 500 species of bacteria inhabit the oral cavity [16].An October 2001 report by the World Health Organization defines probiotics as "live microorganisms which when administered in adequate amounts confer a health benefit on the host" [26].The obvious role of the microbiome and its relation with chronic disease processes is not fully understood. However, its well known that destruction of the local microbiota can result in overgrowth of pathogens and increased risk of infections [16].In recent years, probiotics attract scientists’ attention due to their useful role for the gastrointestinal tract, vaginal mucosa, urinary tract, skin, and oral cavity. It is known that the investigated probiotics are mostly members of intestinal microbiota. Due to the knowledge of probiotics as fermentation factors in the gastrointestinal tract, it became obvious that probiotics also influence the immune system. This knowledge has opened many possibilites in the development of probiotics for therapeutic purposes.It is known that the digestive tract is populated by a huge number of microorganisms that perform many functions. Gut bacteria are capable of fermenting nutrients into digestible forms, including short-chain fatty acids, which have anti-inflammatory and immunomodulatory effects. The key role of the microbiota in host homeostasis has led to the need to investigate its role in the development of many inflammatory diseases of the digestive tract, including esophagitis and Barrett's metaplasia, ulcerative colitis, Clostridium difficile colitis, pancreatic and liver diseases [14].A microbiome is the genetic material of all resident microorganisms (commensal, symbiotic, pathogenic) that inhabit a given niche and function as an organized community [16].Lechien J.R. et al suggest that laryngopharyngeal microbiota is under the influence of various factors that can damage laryngeal mucosa. So, an associated signs and symptoms have a thorough impact on the patient. Patients with normal microbiota are less susceptible to developing symptoms and signs associated with LPR than patients with altered microbiota [4].Akkermansia muciniphila is a beneficial gut commensal. A study by Huck O. et al. evaluate the effect of these bacteria due to anti-inflammatory properties on inflammation forced by Porphyromonas gingivalis. In a white mouse model, scientists induced an experimental model of periodontitis and calvarial abscess by infection with P. gingivalis. Supplementation with A. muciniphila reduced inflammatory cell infiltration. Treatment with A. muciniphila resulted in decreased alveolar bone loss. In vitro studies, an addition of A. muciniphila to P. gingivalis -infected bone marrow macrophages increased the amount of anti-inflammatory IL-10 and decreased IL-12. This study suggests that administration of A. muciniphila may act as a protective factor and therapeutic agent to periodontal treatment [12].The work of Sereg-Bahar M. et al. noted the laryngopharyngeal reflux as the predisposing factor on the development of laryngeal carcinoma. Colleagues compared pH, bile acid levels, total pepsin, and pepsin enzymatic activity in saliva in 30 patients with T1 laryngeal carcinoma and in a group of 34 healthy volunteers. The levels of total pepsin and bile acids was estimated in both groups and it was noticed that the groups differed significantly in in the saliva sample. Higher levels of total pepsin and bile acids were found in the cancer group. The findings suggest that laryngopharyngeal reflux plays a crucial role in the development of laryngeal carcinoma. There was found the higher levels of typical laryngopharyngeal components in the saliva of patients with early laryngeal cancer compared with controls [22]. The microbial communities inhabiting the laryngeal mucosa create a stable microenvironment and can influence the health of the human throat. According to Gong H. et al., there is a difference between bacteria in patients with laryngeal cancer and patients from control group. Disruption of microbiota structure may be relevant to laryngeal cancer because the profile of microbiota of the larynx is altered in patients with laryngeal cancer [10].The role of dysbiosis in the development of laryngitis is confirmed by the significant formation of biofilms, which form microorganisms in chronic laryngitis. Several representatives are involved in biofilm formation such as S. aureus, H. influenzae, C. albicans, Moraxella non-liquefa, Propionibacterium acnes, Neisseria meningitis and S. Pneumoniae [7].Guglielmetti S. et al. isolated Streptococcus salivarius strains RS1 and ST3 from the pharynx of healthy volunteers. This strain is turned out to be the prototype of the oral probiotic strain S. salivarius K12. The mechanism of oral probiotics includes bounds to human pharyngeal epithelial cells. This way all three strains effectively antagonized the adhesion and growth of Streptococcus pyogenes. They are also sensitive to various antibiotics commonly used to fight upper respiratory tract infections. Streptococci S. salivarius K12 was selected as a commensal that can work as a potential candidate for pharyngeal probiotics and show a good level of adaptation to the human body. Investigated strain are also have a potential immunomodulatory and anti-inflammatory properties [9].There are three main mechanisms that probiotics can use to intervene with the activity of pathogenic bacteria. The first one is the production of antimicrobial substances such as small molecules, bioactive peptides, bacteriocins. The second way is the creation of a physiological environment that is unfavorable for the pathogen like competing with the nutrients, altering the pH. Finally, probiotics adhere to epithelial cells and this way preclude pathogen interaction with surface molecules. Moreover, live probiotics or their metabolites can interact with antigen presenting cells and T-cells and assign them to regulate immune function. Thereby probiotics balance immune homeostasis through various pro-inflammatory and anti-inflammatory signaling pathways [12]. Streptococcus salivarius is the primary and prevailing colonizer of human oral cavity, the stomach and jejunum, and does not cause infections in healthy individuals.Streptococcus salivarius K12 (BLIS K12) is a probiotic strain that antagonise the increase of pathogenic microbs. Bacteriocins are type of peptides that are synthesized in bacterial ribosomes and have an antimicrobial effect. Bacteriocins can harm or inhibit bacterial strains closely associated or non-related to produced bacteria, but will not kill the bacteria themselves due to specific immunity proteins [20].The bacteriocins produced by Streptococcus salivarius K12 strain inhibit not only Streptococcus pyogenes, but also other oral bacterial pathogens that cause pharyngotonsillitis, acute otitis media and halitosis.S. salivarius K12 produces bacteriocin-like inhibitory substances (BLIS): the lantibiotics salivaricin A2 and salivaricin B. In vitro studies show that lantibiotics demonstrate an effect against Streptococcus pyogenes and various bacterial species.Variety tissues and blood cells generate Interleukin-8 (IL-8) that is a chemoattractant cytokine. In contradiction to other cytokines, IL-8 has a particular target specificity for the blood xells, especially for the neutrophils. Interleukin-8 angages and activates neutrophils in inflammatory areas. However, K12 strain interferes with IL-8 synthesis and demonstrates immunomodulatory properties, suppressing IL-8 secretion and inhibiting the NF-kB pathway [3].According to Di Pierro F. et al. the episodes of pharyngotonsillitis in children and adults with recurrent streptococcal infection was reduces by applying BLIS K12 [6].Despite the frequent occurrence, there is lack of the literature devoted to the assessment of microbiota of the pharynx in chronic pharyngitis. Our research included an investigation of these question.Aim of our study is to determine the composition of the oropharyngeal microbiota in patients with chronic pharyngitis and to evaluate the effectiveness of the oral probiotic Streptococcus salivarius K12 in the treatment of this category of patients.
2. Materials and Methods
There were examined 30 patients with chronic pharyngitis (CP) caused by GERD. Of these, there were 10 (33,3%) men and 20 (66,6%) women. The study included taking a patient’s complaints and history, ENT examination, general clinical examination, collection of material from the posterior pharyngeal wall for bacteriological examination and determination of sensitivity to antibiotics, as well as esophagogastroduodenoscopy (EGDFS).The main purpose of an ENT examination was to identify signs of chronic pharyngitis. Based on the nature of the changes developing in the mucous membrane of the pharynx, they are divided into: catarrhal, atrophic and hypertrophic chronic pharyngitis.The constant diffuse hyperemia, edema, vasodilation of the pharyngeal mucosa, and hypersecretion of the mucous glands are observed in catarrhal pharyngitis. The mucous membrane of the pharynx looks thinned, dry, pale pink in color with a dull tint, covered in places with crusts and viscous mucus in atrophic pharyngitis. Injected vessels may be visible on the shiny “varnish” surface of the mucous membrane. Pharyngoscopy reveals foci of hyperplastic lymphoid tissue on the posterior wall of the pharynx or enlarged tubopharyngeal ridges and superficial branching veins in the hypertrophic form of CP.The clinical picture of CP is not characterized by an increase in temperature and a significant deterioration in general condition. In this case, usually the paucity of objective findings does not correspond to the severity of subjective symptoms that bother patients [18].The study group included patients who complained of soreness, rawness, dryness and awkwardness in the throat when swallowing, and the sensation of a foreign body that does not interfere with food intake, but forces frequent swallowing movements.The exclusion criteria were the following: acute respiratory inflammatory diseases; acute inflammatory process in the paranasal sinuses, pharynx or hypopharynx; diseases of the nose and paranasal sinuses; children under 18 years of age, pregnancy and lactation.Statistical processing of the data was performed using the variation statistics method with a confidence interval of 95%.Bacteriological examination of the posterior pharyngeal wall was carried out by collecting a smear and determining the composition of the microbiota using the Matrix-assisted laser desorption/ionization (MALDI) method, which high sensitivity is equal to 1 femtomole. Mass spectrometry which is known as the soft ionization option is applied in MALDI method. The investigation includes several stages. Firstly, test material pre-mixes with the matrix. Then this substance construction is pulsed by irradiation of the laser that is usually made use of compound with a time-of-flight mass analyzer. There is one another equipment so called time-of-flight analyzers (TOF-MS) which is broadly applied for genus- and species-specific identification of microorganisms and in laboratories for as diagnostic tool of infectious diseases. MALDI-TOF method has several advantages in microorganisms’ identification. Despite the fact that various research methods including bacteriological, immunological and molecular genetics has a high spread and popularity, the MALDI method showed high productivity and efficiency. Moreover, MALDI-TOF method has an advantage by low price. Standardized MALDI-TOF MS techniques allow to identify correctly most significant bacteria for clinicians. A number of features can be explored during this method. They are as follow: the spectral characteristics of a considerable number of protein molecules and predominantly ribosomal proteins, which are the distinctive index of a specific microorganism. Finally, MALDI-TOF MS technology can be used for exhaustive yeast identification.Esophagogastroduodenoscopy (EGDFS) was performed for all patients using a standard technique on PENTAX EG-290 flexible fiber endoscope under local anesthesia with Lidocainum 10%. During the examination the condition of mucous membrane of esophagus, stomach and duodenum as well as the function of cardiac and pyloric sphincters were assessed. The size and location of destructive changes of the mucous membrane (erosions, ulcers) were evaluated. There are different endoscopic classifications of reflux disease implemented in clinical usage. The most famous one is Savary-Miller (1977) classification. In accordance to this classification there are 4 degrees of esophagitis depending on the severity of manifestations and the presence of complications of GERD. The Hetzel classification (1988) is also based on 4 degrees of prevalence of the lesion. Lundell LR et al. reported that in 1994 oesophagitis was classified according to endoscopic findings by the international working group, supported by the World Organization of Gastroenterology. Due to this classification, video recording and endoscopic photographs endoscopists got an opportunity to identify mucosal breaks restricted to the tops of mucosal creases, abnormal changes extending along the entire circumference of the esophagus and complicated esophagitis in the form of stricture and columnar lined oesophagus [28].In 2002, a clinical classification of GERD put forward. Scientists at the World Congress of Gastroenterology in Los Angeles declared to differentiate non-erosive reflux disease, erosive esophagitis and Barrett's esophagus. In our investigation the degree of damage of the esophageal mucosa was defined according to classification of M.Savari and the clinical-endoscopic classification adopted at the IX European Gastroenterological Week in Amsterdam [1,4].Non-erosive reflux disease is suspected in cases when there is a presence of clinical symptoms and data of pH-metry of the esophagus in the absence of any endoscopic founding. EGDFS allows to note a non-erosive reflux disease as catarrhal esophagitis. Pathological GER may be confirmed in patients with reflux and the following criteria: pH in the esophagus should be less than 4.0 or more than 7.0, lasting longer than 5 minutes, more than 50 episodes during the day, with a total duration of more than 1 hour and existing for at least 3 months.Literature data shows that the incidence of non-erosive reflux disease is more than 60% of all cases of GERD, while 37% cases were noted as erosive esophagitis with the signs of erosive or erosive-ulcerative defects in the mucosa of esophagus. Additionally, in assessment of clinical symptoms and quality of life of patients with non-erosive reflux disease and erosive or erosive-ulcerative esophagitis scientists declare a comparability in both groups.
3. Results
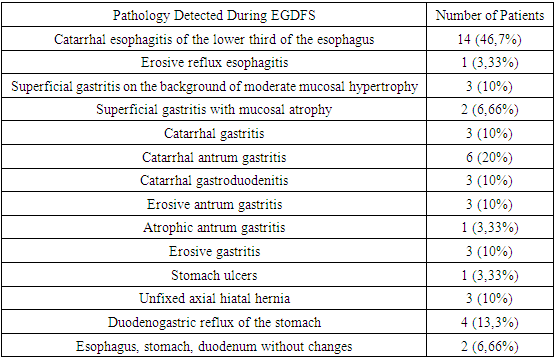
During pharyngoscopy, 2 (6.66%) patients were diagnosed with chronic atrophic pharyngitis, 1 (3.33%) patient with chronic hypertrophic pharyngitis, and 28 (93.3%) with chronic catarrhal pharyngitis.The pathologies of the esophagogastroduodenum were identified based on the EGFDS examination (see Table 1).Table 1. Pathology detected during EGDFS
 |
| |
|
An exhaustive study can lead to the established diagnosis of GERD. However, many scientists approve that even distinctive complaints like dysphonia, pain and/or burning in the throat, mucus flow down the nasopharynx, chronic cough etc. can be sufficient for reflux disease diagnosis. Traditional methods for diagnosing GERD include 24-hour pH-metry, polypositional X-ray examination, endoscopy with biopsy of the mucous membrane of the distal esophagus, manometry, and electromyography of the esophagus [1]. However, GERD can be diagnosed not only using traditional methods but also clinically using questionnaires and taking anamnesis. Researchers assert that at about 50-60% of cases the visible (endoscopic and radiological) changes denote the absence of the disease [1,4].According to microbiological data, the growth of microorganisms such as St.aureus, St.haemoliticus, Str.viridans, Klebsiella pneumoniae, Str.pneumonia, Gemella haemolysans, Enterococcus spp., Enterobacter aeruginosa, Candida albicans was observed on the posterior wall of the oropharynx. The number of detected microorganisms ranged from 105-106 CFU. In 2 cases (6,66%) a combination of several microorganisms was detected: St.aureus and Klebsiella pneumoniae in the first case, and Str.pneumonia and Candida albicans in the second case. The microbiota distribution was as follows: St.aureus - 17 (56.7%), St.haemoliticus - 3 (10%), Str.viridans - 3 (10%), Klebsiella pneumoniae - 4 (13.3%), Str. pneumonia - 1 (3.33%), Gemella haemolysans - 1 (3.33%), Enterobacter aerogenes - 1 (3.33%), Enterococcus spp. – 1 (3.33%), Candida albicans – 1 (3.33%) cases (see Fig. 2).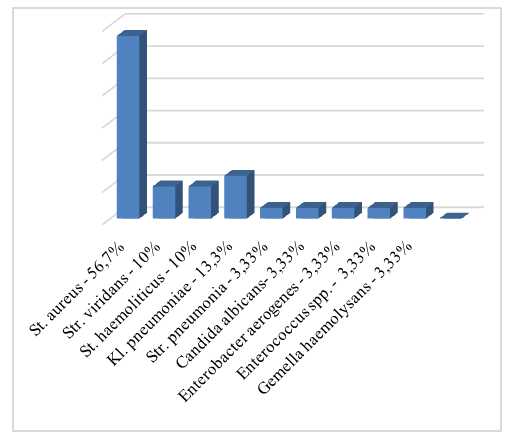 | Figure 2. Distribution of microorganisms according to the results of bacterial investigation before treatment |
All patients were prescribed a standard therapy for GERD by gastroenterologist. Drug therapy for GERD included the use of proton pump inhibitors as a first-line drugs and prokinetics that affect gastrointestinal motility.It is well known that hydrochloric acid plays a crucial role in damaging the mucous of the esophagus during reflux episodes. Healthy person can have reflux episodes no more than 1 hour per day while patients with GERD complain of low pH below 4 for about 4-14,5 hours per day. Due to suppressive effect on parietal cells’ activity and hydrochloric acids’ secretion PPI have been recommended for about 10-15 years for patients with GERD. Parietal cells have a H+, K+-ATPase enzyme on the apical membrane and produce a hydrochloric acid via this enzyme. Activated H+/K+-ATPase molecules integrate in the membranous secretory tubules in parietal cell. On the next stage hydrogen ions are passed from the cell into the glandular lumen, replacing them for potassium ions of the extracellular gap. This process is provided by the energy of H+/K+-ATPase predating the release of chlorine ions from the cytoplasm of the parietal cell. This way the lumen of the secretory tubule of the parietal cell became filled with hydrochloric acid.PPI block the H+/K+-ATPase and the mechanism of hydrochloric acid formation mentioned above linking with molecules of cysteine of the enzyme. This way the synthesis of hydrochloric acid reduces. However, parietal cells are able to return to the enzyme synthesis and hydrochloric acid secretion after 18 hours. This interval supplies an appropriate pH for the management of symptoms of GERD and PPIs show a high effect only in the parietal cells. In this matter, PPIs are used for treatment of GERD for at least 8 weeks with the prolongation of intake for 6 months to 2 years. An advanced generation of PPIs including rabeprazole, esomeprazole, tenatoprazole, can be prescribed in clinical practice and treatment of GERD [1,4,9,29].In our work patients with confirmed GERD received proton pump inhibitors at a dose of 20-40 mg/day as prescribed by a gastroenterologist.Topical therapy in the form of antiseptic gargles and oral probiotic Streptococcus salivarius K12 for 2 months were recommended. The results were evaluated before and after treatment.In our investigation patients took an oral probiotic containing Streptococcus salivarius K12 strains. The probiotic had to be taken once daily before bed. A smear from the posterior pharyngeal wall was taken again 2 months after treatment. According to our data, the titer of detected pathogenic and opportunistic microbiota decreased from 105 – 105 to 102 – 103 CFU after 2 months of treatment with an oral probiotic. The following changes were observed in the microbial composition of the posterior pharyngeal wall after treatment: St.aureus was found in 12 (33.3%) cases, Str.viridans - in 1 (3.33%), Klebsiella pneumonia - in 2 (6.66%) patients (see Fig. 3).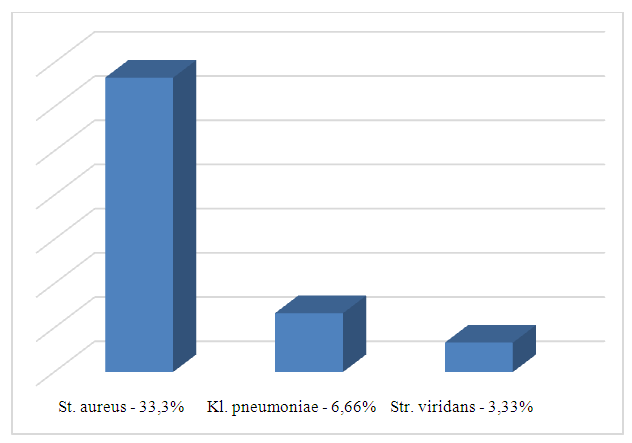 | Figure 3. Distribution of microorganisms according to the results of bacterial investigation after treatment |
There was found a specimen of normal microbiota in the pharynx swabs in 15 (50%) patients after treatment.
4. Conclusions
CP is accompanied by changes in upper gastrointestinal tract confirmed by EGDFS. The examination of esophagus and stomach can be valuable in patients suffering of CP. Also, a modification of the microbiota of the oropharynx with a predominance of opportunistic microorganisms in quantities exceeding the norm was determined. The use of oral probiotics in the treatment of chronic pharyngitis seems promising. The oral probiotic Streptococcus salivarius K12 affects the composition of the microbiota and helps to support a normal amount of oropharyngeal microbiota. We found changes both in microbial composition and in the amount of the microbiota of the posterior pharyngeal wall after treatment.
References
| [1] | Akhmatkhodzhaev A.M. Clinical, pathogenetic and therapeutic aspects of gastroesophageal reflux disease with broncho-obstructive complications - Diss. pp. 27-29, 34-36. |
| [2] | Barry DW, Vaezi MF. Laryngopharyngeal reflux: More questions than answers. Cleve Clin J Med. 2010 May. |
| [3] | Bickel M. The role of interleukin-8 in inflammation and mechanisms of regulation. J Periodontol. 1993 May; 64 (5 Suppl): 456-60. PMID: 8315568. |
| [4] | Bordin D.S. How to choose a proton pump inhibitor for a patient with GERD? // Experiment. and wedge. gastroenterol. - 2010. - No. 2. - P. 53-58. |
| [5] | Carlsson R., Dent J., Bolling-Sternevald E. The usefulness of a structured questionnaire in the assessment of symptomatic gastroesophageal reflux disease // Scand J Gastroenterol. – 1998. – Vol. 33. – Р. 1023-1029. |
| [6] | Di Pierro F. et al. Effect of administration of Streptococcus salivarius K12 on the occurrence of streptococcal pharyngo-tonsillitis, scarlet fever and acute otitis media in 3 years old children. Eur Rev Med Pharmacol Sci. 2016 Nov. |
| [7] | Fekete S, Szabó D, Tamás L, Polony G. A mikrobiom szerepe a fül-orr-gégészetben [The role of the microbiome in otorhinolaryngology]. Orv Hetil. 2019 Sep; 160(39): 1533-1541. Hungarian. doi: 10.1556/650.2019.31451. PMID: 31544493. |
| [8] | Fock K. M., Teo E. K., Ang T. L. et al. Rabeprazole vs esomeprazole in non-erosive gastro-esophageal reflux disease: a randomized, double-blind study in urban Asia // World J Gastroenterol. – 2005. -№ 11. – Р. 3091-3098. |
| [9] | Guglielmetti S, Taverniti V, Minuzzo M, Arioli S, Stuknyte M, Karp M, Mora D. Oral bacteria as potential probiotics for the pharyngeal mucosa. Appl Environ Microbiol. 2010 Jun; 76(12): 3948-58. doi: 10.1128/AEM.00109-10. Epub 2010 Apr 23. PMID: 20418429; PMCID: PMC2893495. |
| [10] | Gong H, Shi Y, Xiao X, Cao P, Wu C, Tao L, Hou D, Wang Y, Zhou L. Alterations of microbiota structure in the larynx relevant to laryngeal carcinoma. Sci Rep. 2017 Jul 14; 7(1): 5507. doi: 10.1038/s41598-017-05576-7. PMID: 28710395; PMCID: PMC5511217 |
| [11] | Graham D. Y. Helicobacter pylori is not and never was "protective" against anything, including GERD // Dig. Dis. Sci. - 2003. - Vol.48, №4. - P. 629-630. |
| [12] | Huck O, Mulhall H, Rubin G, Kizelnik Z, Iyer R, Perpich JD, Haque N, Cani PD, de Vos WM, Amar S. Akkermansia muciniphila reduces Porphyromonas gingivalis-induced inflammation and periodontal bone destruction. J Clin Periodontol. 2020 Feb; 47(2): 202-212. doi: 10.1111/jcpe.13214. Epub 2019 Dec 2. PMID: 31674689. |
| [13] | Irwin R. S., Curley F. J., French C. L. Difficult-to-control asthma. Contributing factors and outcome of systematic managements protocol // Chest. – 1993. – Vol. 103. – Р. 1662-1669. |
| [14] | Jetté ME, Dill-McFarland KA, Hanshew AS, Suen G, Thibeault SL. The human laryngeal microbiome: effects of cigarette smoke and reflux. Sci Rep. 2016 Oct 24; 6: 35882. doi: 10.1038/srep35882. PMID: 27775059; PMCID: PMC5075886. |
| [15] | Kang HJ, Im SH. Probiotics as an Immune Modulator. J Nutr Sci Vitaminol (Tokyo). 2015; 61 Suppl: S103-5. doi: 10.3177/jnsv.61.S103. PMID: 26598815. |
| [16] | Lee JT, Kim CM, Ramakrishnan V. Microbiome and disease in the upper airway. Curr Opin Allergy Clin Immunol. 2019 Feb. |
| [17] | Li Z, Huang J, Hu Z. Screening and Diagnosis of Chronic Pharyngitis Based on Deep Learning. Int J Environ Res Public Health. 2019 May 14. |
| [18] | Maev I.V. and others. Bronchopulmonary and oropharyngeal manifestations of gastroesophageal reflux disease. Consilium Medicum. - 2006, - No. 2, - p. 22-27. |
| [19] | Minty M, Canceil T, Serino M, Burcelin R, Tercé F, Blasco-Baque V. Oral microbiota-induced periodontitis: a new risk factor of metabolic diseases. Rev Endocr Metab Disord. 2019 Dec; 20(4): 449-459. doi: 10.1007/s11154-019-09526-8. PMID: 31741266. |
| [20] | Nandurkar S., Talley N. J. Epidemiology and natural history of reflux disease // Baillieres Best Pract Res Clin Gastroenterol. – 2000. – Vol. 5. – Р. 743-757. |
| [21] | Palchuna V. T. // Otorhinolaryngology: national guide — 2nd ed., revised and additional - Moscow: GEOTAR-Media, 2022. |
| [22] | Sereg-Bahar M, Jerin A, Hocevar-Boltezar I. Higher levels of total pepsin and bile acids in the saliva as a possible risk factor for early laryngeal cancer. Radiol Oncol. 2015 Mar 3; 49(1): 59-64. doi: 10.2478/raon-2014-0020. PMID: 25810702; PMCID: PMC4362607. |
| [23] | Shen Y et al. 16SrDNA-Based Detection Technology in Patients with Chronic Pharyngitis to Analyze the Distribution Characteristics of Pharyngeal Bacteria. J Healthc Eng. 2022 Mar 11. |
| [24] | Vakil N. et al. The Montreal Definition and Classification of Gastroesophageal Reflux Disease: A Global Evidence-Based Consensus The American journal of gastroenterology. 2006. |
| [25] | Vasyaeva A.A., Arefieva N.A. Immunotherapy for chronic pharyngitis: indications, results. RMJ. 2010; 30: 1864. |
| [26] | World Health Organization. Probiotics in food: health and nutritional properties and guidelines for evaluation. Food and Agriculture Organization of the United Nations, Rome: Food and Agriculture Organization of the United Nations. 2006. ISBN 92-5-105513-0. OCLC 70928765. Archived from the original on 2023-07-01. Retrieved 2022-10-31. |
| [27] | Yang SC, Lin CH, Sung CT, Fang JY. Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front Microbiol. 2014 May 26; 5: 241. doi: 10.3389/fmicb.2014.00241. Erratum in: Front Microbiol. 2014; 5: 683. PMID: 24904554; PMCID: PMC4033612. |
| [28] | Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GN, Wallin L. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999 Aug; 45(2): 172-80. doi: 10.1136/gut.45.2.172. PMID: 10403727; PMCID: PMC1727604. |
| [29] | Dubinskaya T. K., Volova A.V., Razzhivina A. A., Nikishina E. I. Acid production of the stomach and methods of its determination. Textbook. Moscow: RMAPO, 2004. — 28 p. ISBN 5-7249-0789-5. |





 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML