-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(6): 1673-1679
doi:10.5923/j.ajmms.20241406.44
Received: May 14, 2024; Accepted: Jun. 8, 2024; Published: Jun. 21, 2024

Pathogenetic Role of Coronavirus Infection in the Development of Acute Kidney Injury
Abdurakhimov Abdukhalim, Kakharov Zafar
Department of Anatomy and Clinical Anatomy, Andizhan State Medical Institute, Andizhan, Uzbekistan
Correspondence to: Abdurakhimov Abdukhalim, Department of Anatomy and Clinical Anatomy, Andizhan State Medical Institute, Andizhan, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This review summarizes the results of major clinical and experimental studies on the role of coronavirus infection in the development of kidney damage and available in the databases www.elibrary.ru, www.cyberleninka.ru, and the Google Scholar search engine.
Keywords: COVID-19, SARS-CoV-2, Acute kidney injury, Chronic kidney disease, Kidney damage, Pathogenesis, Nephropathy, Comorbidity
Cite this paper: Abdurakhimov Abdukhalim, Kakharov Zafar, Pathogenetic Role of Coronavirus Infection in the Development of Acute Kidney Injury, American Journal of Medicine and Medical Sciences, Vol. 14 No. 6, 2024, pp. 1673-1679. doi: 10.5923/j.ajmms.20241406.44.
Article Outline
1. Introduction
- Although coronavirus infectious disease (COVID-19) is primarily a respiratory disease, the kidney may be among the target organs of infection with severe acute respiratory syndrome coronavirus 2 (SARS-COV-2). Data on the kidney involvement of COVID-19 patients are still very scarce.However, as more patients are infected worldwide, our understanding of the disease is rapidly evolving [15].Acute kidney injury (AKI) is one of the complications of COVID-19 that determines the prognosis of the disease. The first studies conducted in China showed the frequency of detection of AKI in adult patients before 29% [3] [6] [7] [16]. The incidence of AKI in the general population is continuously increasing and reaches 0.25%, which is comparable to the incidence of acute myocardial infarction. Despite the continuous improvement of therapeutic technologies, primarily methods of renal replacement therapy, there is no significant improvement in the results of AKI treatment. The outcomes of severe AKI variants remain unsatisfactory, and their mortality rate can reach 70% or more [29]. According to I. Cheruiyot et al., obtained as a result of a study in 2020, AKI is associated with a worse prognosis [4]. E.S. Stolyarevich et al. in 2020 conducted clinical and morphological comparisons of the manifestations of renal pathology in 220 adult patients who died from COVID-19 [30]. The morphological substrate of AKI in most cases was acute damage to the tubular epithelium, venous fullness of the organ (in more than half of the patients), as well as the presence of thrombotic microangiopathies. Many authors emphasize that COVID-19 infection can complicate treatment, care and increase mortality in people with underlying kidney disease [7] [16] [20]. Several studies have shown that the development of AKI in COVID-19 is associated with a high risk of death [5] [6] [9]. However, contradictory data are provided on the frequency of AKI, which varied from 0.5 to 37% in different studies [5] [14] [18]. J. Hirsh et al. Signs of AKI were detected in 36.6% of hospitalized patients with COVID and in 89.7% of patients on artificial lung ventilation (ventilator) [9]. In another study, AKI was observed in 27% of hospitalized patients with COVID-19 and was more common in elderly people with concomitant diseases – arterial hypertension and heart failure [5]. However, in some studies, the incidence of AKI in patients with COVID-19 was significantly lower. For example, L. Wang et al. Renal dysfunction was not detected in 116 hospitalized patients [19]. According to a large Chinese national study based on clinical data from 552 hospitals in 30 provinces, autonomous regions and municipalities (n=1099), AKI develops in 0.5% of patients with confirmed COVID-19. It should be noted that this study included patients younger than 60 years old, and most of them had a mild course of the disease [6].Several pathogenic mechanisms have been implicated, including critical hypoxia, infammation and sepsis, haemodynamic changes, acute cardiorenal syndrome, rhabdomyolysis, mitochondrial injury, endothelial dysfunction, microembolism, kidney infarction and use of nephrotoxic drugs [11] [15].Analysis of existing publications showed that older patients and patients with comorbid backgrounds lead the risk group for COVID-19, and the risk of mortality can reach 20% [28]. In such patients, the acquired immune response may be weakened and increasingly compensated by an innate immune response, which leads not only to the elimination of the virus, but also to extensive tissue damage due to the development of a cytokine storm, accompanied by acute respiratory distress syndrome. Cases of COVID-19 infection are more common among patients with aggravated somatic status: cardiovascular disorder and diabetes mellitus [21].According to an analysis of more than 70,000 cases of COVID-19 registered in Wuhan, mild infection was observed in 81% of patients, severe in 14% and extremely severe (respiratory failure, multiple organ failure and/or septic shock) in 5% [2] [25]. Overall, the mortality rate for patients with confirmed SARS-CoV-2 infection was 2.3%, but it was significantly higher among older people (8.0% aged 70-79 years and 14.8% aged ≥80 years) and especially among patients who were in critical condition (49.0%). Higher lethality was also found in the presence of cardiovascular diseases (10.5%), diabetes mellitus (7.3%), chronic lung diseases (6.3%), arterial hypertension (6.0%) and malignant neoplasms (5.6%).
2. Materials and Methods
- For the study the role of the new coronavirus infection in the development of acute kidney injury, we studied 58, 30 sources of them included in this review. The keywords were "Acute kidney injury", "Chronic kidney disease", "COVID-19", "SARSCOV-2".
3. Results
- The Extent of Kidney Injury in COVID-19The pathophysiological mechanisms leading to acute kidney injury (AKI) during COVID-19 infection are unclear, but may be due to the effects on renal tubules and endothelial cells that occur during cytokine storm and subsequent damage to the microvasculature as a result of disturbances in the system of blood clotting (Fig. 1) [12] [17].
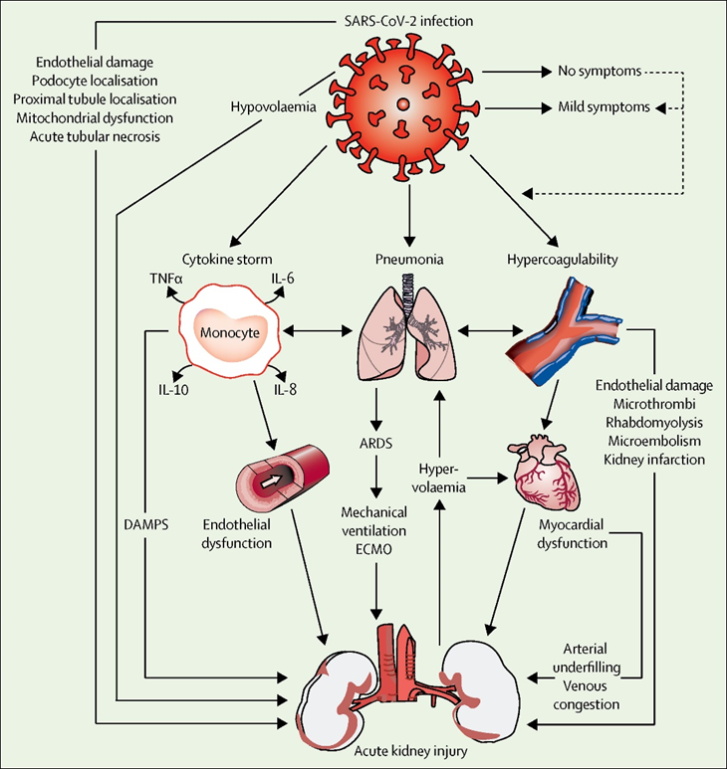 | Figure 1. Mechanism of kidney injury in COVID-19 |
|
4. Discussion
- Acute hyperglycemia has been shown to increase ACE2 expression in cells, which may facilitate viral cell entry. However, chronic hyperglycemia, as already noted, reduces the expression of ACE2, making cells vulnerable to the inflammatory and damaging effects of the virus. The interaction between COVID-19 and diabetes may be bidirectional. As noted above, SARS-CoV-2 enters human cells through ACE2. ACE2 is widely expressed in the liver and pancreas, and its deficiency plays a role in the development of insulin resistance and impaired insulin secretion. After endocytosis of the viral complex, ACE2 expression decreases, leading to two types of consequences. First, entry of SARS-CoV-2 into pancreatic islet cells may directly exacerbate beta cell damage. Second, inhibition of ACE2 after viral entry may lead to unopposed angiotensin II production, which impairs insulin secretion. These data suggest that infection may cause the development of diabetes or, at a minimum, severe stress hyperglycemia. The fact that COVID-19 infection causes hyperglycemia in people without pre-existing diabetes has already been documented by some researchers [24].It is known that diabetes mellitus (DM) complicates the course of many diseases, as it affects almost all systems of the human body [24]. As the practice of 2020 has shown, this phenomenon also applies to infectious diseases [13]. According to WHO recommendations, patients with diabetes are a category of patients at high risk of severe COVID-19. There is information regarding evidence of the cause of the unfavorable course of coronavirus infection in this group of patients [24]. The main argument is the important role of hyperglycemia, which is observed in patients who do not control the course of diabetes [28].It is known that hyperglycemia causes dilatation of the afferent arterioles of the renal glomeruli, their hyperperfusion and an increase in GFR through mechanisms such as increased synthesis of growth hormone, insulin-like growth factor, glucagon, prostaglandins, nitric oxide. In addition to the listed factors, an increase in GFR is caused by increased synthesis of sorbitol, an increase in extracellular fluid volume with increased secretion of atrial natriuretic peptide, glucosuria, a decrease in insulin levels and other mechanisms that are also a consequence of hyperglycemia. Activation of the intrarenal renin-angiotensin system (RAS) plays an important role in the pathogenesis of DN. Hyperglycemia activates this system (by activating protein kinase C, isoform β2), which is one of the most important mechanisms of damage to the renal microvasculature and the formation of DN.
5. Conclusions
- In summary, kidney injury in patients with coronavirus disease is common and can range from proteinuria and hematuria to acute kidney injury, which is associated with high mortality and serves as an independent risk factor for hospital death from all causes in patients with COVID-19. The pathophysiology and mechanisms of kidney injury in patients with COVID-19 are not fully understood and appear to be multifactorial [26]. No specific glomerular pathology was observed in the kidneys during COVID-19. At the same time, acute tubular necrosis was detected in all samples of renal tissue [10] [26]. In patients with SARS-CoV-2 infection, the prevalence of kidney damage is high and usually leads to a poor prognosis, increasing the importance of nephroprotection. New evidence suggests that CKD (Chronic kidney disease) or previous AKI first diagnosed during hospitalization should be recognized as risk factors for severe COVID-19.
Conflict of Interest
- The authors declare no conflicts of interest or special funding for the current study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML