Egamberdieva Mashkhura
Samarkand State Medical University, Samarkand City, Republic of Uzbekistan
Correspondence to: Egamberdieva Mashkhura, Samarkand State Medical University, Samarkand City, Republic of Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The fixed combination of brinzolamide 1% and brimonidine 0.2% (FCB), was the first fixed combination produced that did not include a beta blocker. It can be used alone or together with timolol, PG analogues and their combinations. As far as we know from the literature, no studies have been conducted in Uzbekistan to assess the effectiveness and safety of this fixed combination for IOP control. The aim of this study was to investigate the effect of lowering IOP, side effect profile, and treatment adherence in glaucoma patients receiving FCBB therapy.
Keywords:
Glaucoma, Treatment, Medications
Cite this paper: Egamberdieva Mashkhura, Evaluation of the Use of a Fixed Combination of Brinzolamide-Brimonidine in Maximum Drug Therapy, American Journal of Medicine and Medical Sciences, Vol. 14 No. 6, 2024, pp. 1654-1659. doi: 10.5923/j.ajmms.20241406.39.
1. Introduction
Glaucoma is a chronic neuropathy of the optic nerve characterized by progressive atrophy of the optic disc, degeneration of retinal ganglion cells and typical loss of the visual field [1]. Since many glaucoma patients have severe vision loss at diagnosis, and the damage caused is irreversible, early diagnosis and accurate and objective monitoring of progression are important. Intraocular pressure (IOP) is the main parameter used in the diagnosis, treatment, follow-up and classification of glaucoma. IOP is the most well-known risk factor for glaucomatous damage and is considered the most important modifiable factor for preventing progressive glaucomatous lesion [2]. Multicenter large-scale clinical studies have shown that lowering IOP can prevent or delay the progression of visual field defects caused by glaucoma and preserve visual function [4]. Determining the target IOP is important in the treatment of glaucoma. The initial treatment of glaucoma is aimed at reducing increased intraocular pressure with one or more topical anti-glaucoma drugs to reduce the production of watery moisture and/or increase the outflow of watery moisture [6].Anti-glaucoma agents with various mechanisms of action are available. Beta-blockers and carbonic anhydrase inhibitors reduce intraocular pressure by reducing the production of watery moisture; prostaglandin analogues (PG) enhance uveoscleral and trabecular outflow; and alpha-2 agonists reduce IOP both by reducing the secretion of watery fluid and by increasing uveoscleral blood flow [4]. In a significant proportion of glaucoma patients, IOP cannot be reduced to the target range with a single drug, and a second or third drug must be added to treatment to control glaucoma. With maximum drug therapy, the use of three or more different classes of anti-glaucoma agents is required to achieve a sufficient reduction in IOP [7]. In the treatment of glaucoma, two-, three- or four-component drugs can be used alone or as fixed combinations of two drugs in one bottle to facilitate use and reduce side effects associated with preservatives. It is known that the use of fixed combinations is more convenient and beneficial than the use of several drugs in terms of patient compliance with the treatment regimen, dosage, ease of use and side effects [9]. Commonly used combinations include the topical beta-blocker 0.5% in combination with a PG analog, an alpha-adrenergic receptor agonist, or a local carbonic anhydrase inhibitor. Although fixed combinations containing beta-blockers usually provide effective reduction of IOP, they should not be prescribed to patients with conditions such as asthma, chronic obstructive pulmonary disease, sinus bradycardia, impotence and depression [10].The fixed combination of brinzolamide 1% and brimonidine 0.2% (FCBB), was the first fixed combination produced that did not include a beta blocker. It can be used alone or together with timolol, PG analogues and their combinations. As far as we know from the literature, no studies have been conducted in Uzbekistan to assess the effectiveness and safety of this fixed combination for IOP control. The aim of this study was to study the effect of lowering IOP, side effect profile, and treatment adherence in glaucoma patients receiving FCBB therapy.
2. Materials and Methods
Patient data were retrospectively analyzed. Written informed consent was obtained from the patients who participated in the study. The study sample included patients who received various anti-glaucoma drugs for glaucoma and ophthalmohypertension (OGT) and were transferred to the maximum regimen of drug therapy to achieve the target IOP by adding FCBB for reasons such as non-compliance with the regimen of 3-4 different drugs for the treatment of glaucoma. All patients received a fixed combination of brinzolamide 10 mg/ml (1%) and brimonidine 2 mg/ml (0.2%) twice daily. Patients were divided into four groups depending on the anti-glaucoma treatment used before switching to FCBB therapy:Group 1: fixed combination of brinzolamide/beta-blocker + therapy with PG analogues.Group 2: brinzolamide + fixed combination therapy with beta-blocker/PG.Group 3: brimonidine + a fixed combination of brinzolamide/beta-blocker + therapy with PG analogues.Group 4: brimonidine + brinzolamide + fixed combination therapy with beta-blockers/PG.During the initial examination of all patients, a detailed anamnesis was collected, followed by measurement of the maximum corrected visual acuity, examination of the anterior segment of the eye using a slit lamp, examination of the fundus with pupil dilation, measurement of IOP using a Goldman applanation tonometer, angle examination using a 4-mirror goniol lens, measurement of the central thickness of the cornea using an ultrasound pachymeter, visual field analysis and measurements of the retinal nerve fiber layer and ganglion cell complex using optical coherence tomography in the spectral region (Carl Zeiss Meditec, Dublin, California, USA). Patient records were reviewed to collect the following data:The data was analyzed using the SPSS software package for Windows version 11.5 (SPSS Inc, Chicago, Illinois, USA). Descriptive statistics were expressed as the mean ± standard deviation for normally distributed variables and as a number (n) and percentage (%) for nominal variables. After importing the IOP values into the SPSS program, the t-test for paired samples was used to determine the statistical significance of the differences between the baseline level and the values measured after 1, 3 and 6 months to determine the effect of drug use in three different periods. The results with p<0.05 were considered statistically significant in statistical analysis.
3. The Results of the Study
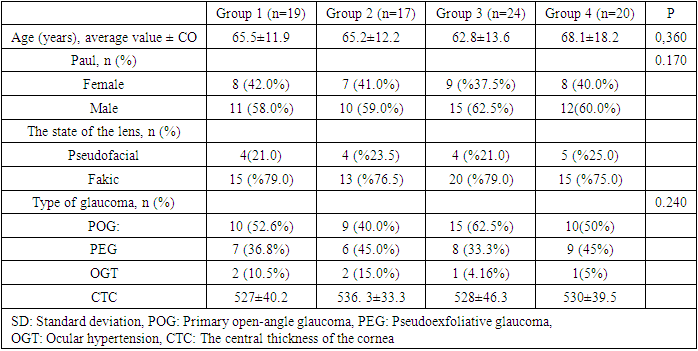
A total of 80 patients were included in the study. Four patients stopped taking FCBB after 1 month of follow-up due to side effects and were not included in the analysis. The average follow-up period after the start of FCBB for all patients was 8.7 ± 4.5 months (range: 5-11). Demographic and clinical characteristics of patients in four groups are shown. (Table 1) There were 19 patients in group 1 (23.75%), 17 in group 2 (21.25%), 24 in group 3 (30.0%), and 20 in group 4 (25.0%). There are 32 women (40.0%) and 48 men (60.0%). There were no statistically significant differences between the groups in terms of gender distribution (p>0.05). The average age of all patients was 65.4±16.6 years (range: 18-90 years). The average age did not significantly differ between the groups (p>0.05). In the general group of patients, 43 (53.75%) patients were diagnosed with primary open—angle glaucoma (POAG), 30 (37.5%) with pseudoexfoliative glaucoma (PEG) and 6 (7.5%) with OGT. There was no statistically significant difference between the groups by type of glaucoma (p>0.05) (Table 1).Table 1
 |
| |
|
The average IOP in all patients in the study before treatment was 21.1±4.8 mmHg (range: 17-25) and decreased to 18.6±3.7 mmHg after 1 month; 18.3±3.4 mmHg after 3 months; and 18.0±3.5 mmHg after 6 months (p<0.001). The IOP values of patients in all groups before FCBB and 1, 3 and 6 months after FCBB are shown in Table 2.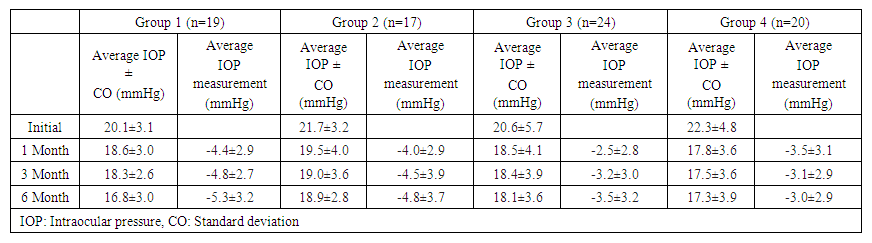 | Table 2. Average IOP and change in IOP values after treatment with a fixed brinzolamide/brimonidine combination, by group |
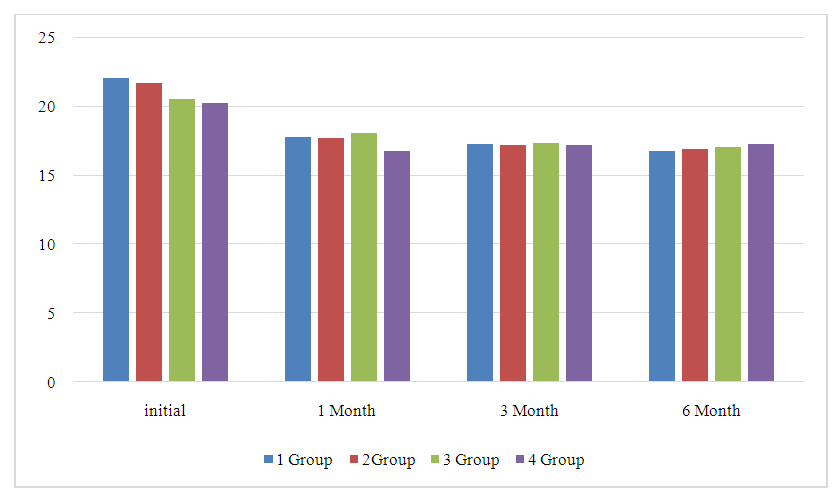 | Figure 1. Average values of intraocular pressure (IOP) during follow-up during treatment with a fixed combination of brinzolamide/brimonidine, in patients by group |
The average changes in IOP in patients in four groups after the start of FCBB are shown in Figure 2. Among all patients in the study, the average change in IOP compared to the baseline level was -3.5±3.4 mmHg after 1 month, -3.8±3.5 mmHg after 3 months, -4.1±3.8 mmHg 6 months after the start of FCBB. The average percentage changes in IOP from baseline to 1 month after FCBB in all patients and in four groups are shown in Fig.2. In all patients, the average IOP values decreased by 16.6% after 1 month, 18% after 3 months and 19.3% after 6 months compared with the baseline level after FCBB. (Fig. 2). The average changes in IOP and a percentage decrease after 6 months in the diagnosis of glaucoma can be seen in Figure 3. After 6 months, the average change in IOP compared to the baseline level was -4.1±3.4 mmHg (19.5%) in patients with POAG, -4.0±3.2 mmHg (18.9%) in patients with PEG and -4.1±3.3 mmHg (19.3%) in patients with GGT. There were no statistical differences between the types of glaucoma in terms of changes in IOP or percentage decrease compared to baseline.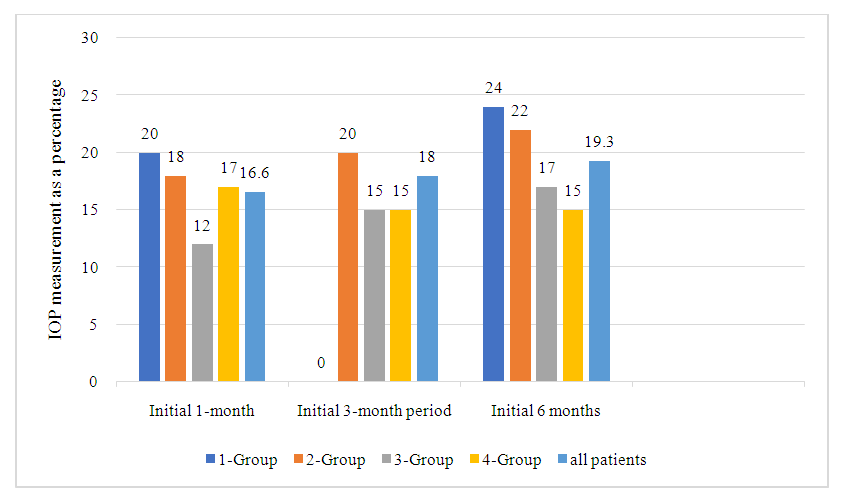 | Figure 2. Average percentage change in intraocular pressure (IOP) during follow-up during treatment with a fixed brinzolamide/brimonidine combination, in all patients and by group |
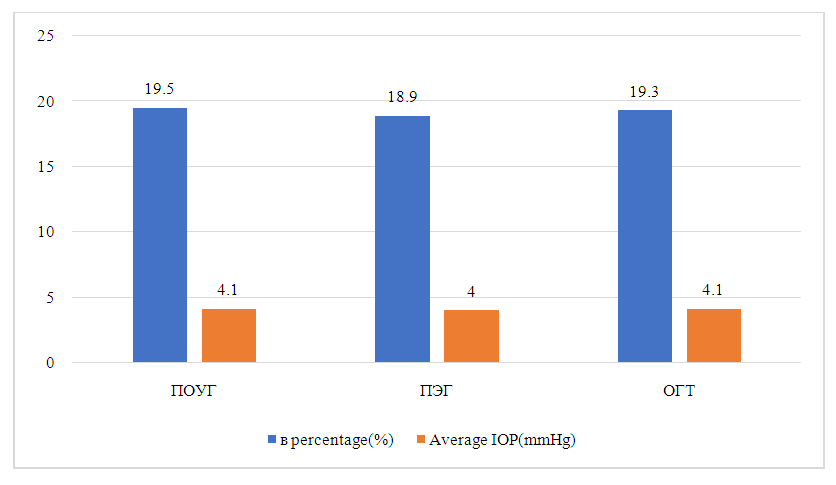 | Figure 3. Average change in intraocular pressure (IOP) and percentage change after 6 months according to the diagnosis of glaucoma. POAG: primary open-angle glaucoma, PEG: pseudoexfoliative glaucoma, GGT: ocular hypertension |
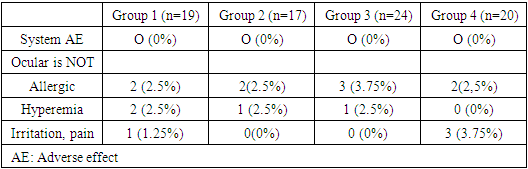
Prior to FCBB, patients of groups 1 and 2 used a total of two local anti-glaucoma drugs, and patients of groups 3 and 4 used three local anti-glaucoma drugs. The average number of topical ant glaucoma drugs used in all patients was 2.5±0.6 before FCBB and decreased to 2 after the start of FCBB (p<0.001).Drug side effects from the eyes were observed in 15 (18.75%) patients treated with FCBB continuously during the 6-month follow-up period, and 13 (16.25%) of these patients stopped treatment due to side effects. FCBB therapy was not discontinued in 4 patients with mild eye irritation. The most common side effect was an allergic reaction to the drug during the first 2 weeks of FCBB use (n=8.10.0%), accompanied by conjunctival hyperemia (n=4, 5.0%) and eye irritation/discomfort (n=4, 5.0%) (Table 3). There was no statistical difference between the groups in the frequency of adverse events (p>0.05). Two patients who had not previously taken brimonidine reported systemic hypotension after starting FCBB.Table 3. Side effects observed after treatment with a fixed brinzolamide/brimonidine combination, by group
 |
| |
|
Currently, the treatment of first-line glaucoma is mainly based on the topical application of one or more anti-glaucoma drugs to reduce IOP. Before resorting to surgical treatment, in order to achieve the target IOP, it is necessary to start treatment with several drugs. Studies have shown that the use of more than two drops per day negatively affects the patient's compliance with the treatment regimen and, as a result, the success of treatment. [12] Thus, drugs acting with fewer drops, or fixed combinations containing two drugs in one vial, are preferred. A combination of fixed doses of 1% brinzolamide (carbonic anhydrase inhibitor) and 0.2% brimonidine tartrate (alpha-2-adrenergic receptor agonist) was approved by the U.S. Food and Drug Administration in April 2013 as a new treatment option for patients with POAG. or GGT.In our study, the effectiveness of FCBB was studied in 80 patients with POAG, PEG or GGT who required maximum drug therapy. In this study, FCBB with combination therapy with beta-adrenoblocker and PG was prescribed to patients of groups 1 and 2 when triple therapy with beta-adrenoblocker, a PG analog and a carbonic anhydrase inhibitor was insufficient, and a statistically significant decrease in IOP was observed in both groups 1, 3 and 6 months after the change of treatment. In addition, there was a statistically significant decrease in IOP 1, 3 and 6 months after the start of combined therapy with FCBB and beta-blocker/PG in group 3 patients who had previously received therapy in 3 different vials with brimonidine, a combination of brinzolamide/beta-blocker and a PG analog, and in group 4 patients who had previously received brimonidine, brinzolamide, and combination therapy beta-blocker/PG in 3 different vials. We believe that the significant decrease in IOP observed after switching to FCBB in groups 3 and 4 who received four-component antiglaucomatous therapy before the start of FCBB may be associated with improved treatment adherence due to fewer vials of the drug and fewer preservatives, as well as a decrease in the ability for drugs to wash each other out.Previous studies in the literature have mainly evaluated the effectiveness of FCBB alone in patients with glaucoma. In several recent studies, patients received the maximum regimen of FCBB drug therapy together with a combination of beta blockers and PG. Lerner et al. [7] evaluated the maximum drug therapy by examining the additive efficacy of FCBB with a fixed combination of in 67 patients with open-angle glaucoma and GGT and observed a statistically significant decrease in IOP in the group to which FCBB was added. Joe and Jean [18] divided patients receiving maximum drug therapy into two groups: triple (dorzolamide-timolol + brimonidine + latanoprost combination) and double (tafluprost/timolol + BBFU combination) maximum therapy. There was no statistically significant difference between the double and triple maximum drug therapy groups in terms of the rate of IOP reduction, but the incidence of ocular side effects such as conjunctival hyperemia and dry eyes was significantly lower in the double maximum therapy group. Similarly, Wy et al. [19] examined patients with POAG who switched from triple maximum drug therapy (dorzolamide/timolol + brimonidine + latanoprost combination) to double maximum drug therapy (tafluprost/timolol combination + FCBB). Although IOP reduction rates were the same in the double and triple maximum drug therapy groups, the incidence of dry eye syndrome was significantly lower in the double maximum therapy group.Reducing IOP is the only glaucoma treatment that has been proven effective in maintaining visual function. The results of the study showed that a decrease in IOP for every 1 mmHg reduces the progression of glaucoma by 10%. In our study, the average decrease in IOP was 4.1 mmHg in the entire group of patients according to data obtained 6 months after the start of FCBB. The largest decrease was 5.3 mmHg in group 1, followed by 4.8 mmHg in group 2 by 3.5 mmHg in group 3 by 3.0 mmHg in group 4. Studies have shown that IOP values are below 18 mmHg. reduce visual impairment. [3] In our study, IOP values measured after 6 months were below 18 mmHg in 82% of patients in group 1, 85% of patients in groups 2 and 3, and 86% of patients in group 4.The number of topical ant glaucoma drugs used in all patients decreased on average from 2.5±0.6 before FCBB to 2 after the start of FCBB. Fewer drops make it easier to use and reduce the number of vials that the patient needs to buy, thereby reducing the cost of treatment. We believe that increased adherence to treatment and a lower likelihood of each other's drugs leaching out when using fewer drops played a role in a significant decrease in IOP after FCBB. After treatment with a fixed combination, side effects such as damage to the surface of the eye and dry eyes associated with the toxic effect of preservatives such as chloride decrease and the tolerability of the drug increases [16].In our study, the incidence of ocular side effects after FCBB was 16.3%. These include allergic reactions, irritation and conjunctival hyperemia, which are known side effects of brinzolamide and brimonidine. No serious side effects or systemic side effects were observed. In previous studies, adverse drug reactions similar to those in our study after FCBB have been reported in the literature [7-20]. As in our study, Lerner et al [7], reported that the incidence of ocular side effects was 11.9% in patients who received maximum drug therapy in the form of FCBB and a beta-blocker/PG analog. In the same study, side effects from the eyes were observed in 7.5% of the control group taking only a combination of beta-blocker/PG analog.Previous literature studies show that the majority of patients receiving FCBB were diagnosed with POAG [19].
4. Conclusions
In contrast, our study included 44 patients with POAG (55.0%) and 30 patients with PEG (37.5%). FCBB provided effective reduction of IOP in both patients with POAG and patients with PEG. After 6 months, IOP decreased compared to the baseline level by 4.12±3.37 mmHg (19.5%) in patients with POAG and by 4.02±3.17 mmHg (18.9%) in patients with PEG, without a statistical difference in the average IOP change or percentage change in the group.A fixed combination of brinzolamide 1% and brimonidine 0.2% provides effective reduction of IOP in patients with POAG, PEG and GGT who need the use of several antiglaucoma drugs. In most patients in our study, FCBB was well tolerated as part of maximum drug therapy, increased treatment adherence by reducing the number of drug containers used, and caused no side effects other than the known ocular side effects of drug components.
References
| [1] | Casson RJ, Chidlow G, Wood JP, Crowston JG, Goldberg I. Definition of glaucoma: clinical and experimental concepts. Clin Exp Ophthalmol. 2012; 40:341-349. [PubMed] [Google Academy] |
| [2] | Peters D, Bengtsson B, Heijl A. Factors associated with a lifetime risk of blindness in open-angle glaucoma. Acta Ophthalmol. 2014; 92: 421-425. [PubMed] [Google Academy] |
| [3] | The authors are not specified. Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between intraocular pressure control and visual field deterioration. AGIS researchers. Am J Ophthalmol. 2000; 130: 429-440. [PubMed] [Google Academy] |
| [4] | European Glaucoma Society. Terminology and recommendations for glaucoma. 3rd ed. Savona: Editrice Dogma Srl; 2008. [Internet] |
| [5] | Hale A., Leske M.S., Bengtsson B., Hyman L., Bengtsson B., Hussein M.; Early manifest glaucoma Research Group. Reduction of intraocular pressure and progression of glaucoma: results of a study of early manifest glaucoma. Arch Ophthalmol. 2002; 120: 1268-1279. [PubMed] [Google Academy] |
| [6] | The authors are not specified. Terminology and Recommendations of the European Glaucoma Society on Glaucoma, 4th edition - Chapter 3: Principles and Treatment Options Supported by the EGS Foundation: Part 1: Preface; Introduction; Glossary; Chapter 3 Principles and Treatment Options. Br J. Ophthalmol. 2017; 101: 130-195. [Free PMC article] [PubMed] [Google Scholar] |
| [7] | Lerner S.F., Oddone F., Lu D.V., Sanso A., Guarro M., Ridolfi A., Hubach D. Maximum drug therapy: fixed combinations of brinzolamide/brimonidine and travoprost/timolol in glaucoma and ocular hypertension. Klin Ophthalmol. 2019; 13:2411-2419. [Free PMC article] [PubMed] [Google Scholar] |
| [8] | Higginbotham EJ, Hansen J, Davis EJ, Walt JG, Guckian A. The durability of glaucoma treatment with a fixed combination compared to several bottles. Curr Med Res Opin. 2009; 25:2543-2547. [PubMed] [Google Academy] |
| [9] | Schwartz G., Burke S., Bennett T., Patel V.D. Commitment and perseverance in glaucoma therapy: brimonidine/timolol compared with dorzolamide/timolol and various combinations of two vials. J Clin Exp Ophthalmol. 2012; 3:1-6. [Google Academy] |
| [10] | Taniguchi T., Kitazawa Yu. The potential systemic effect of topical beta-blockers in the treatment of glaucoma. Kurr Opin Ophthalmol. 1997; 8: 55-58. [PubMed] [Google Academy] |
| [11] | Patel S.K., Spat G.L. Compliance with the treatment regimen of patients prescribing eye drops for glaucoma. Ophthalmic Surgeon. 1995; 26:233-236. [PubMed] [Google Academy] |
| [12] | Vainreb R.N. Compliance with medical treatment of glaucoma. J Glaucoma. 1992; 1:134-136. [Google Academy] |
| [13] | Mallard A. FDA Drug Approval, 2013 Nat Rev Drug Discov. 2014; 13:85–89. [PubMed] [Google Academy] |
| [14] | Kóthy P, Holló G. Practical experience of using a combination of fixed brinzolamide/brimonidine drops in the center of tertiary glaucoma. Int ophthalmol. 2020; 40: 377-383. [PubMed] [Google Academy] |
| [15] | Gandolfi SA, Lim J, Sanseau AC, Parra Restrepo JC, Hamacher T. A randomized trial of brinzolamide/brimonidine versus brinzolamide plus brimonidine in open-angle glaucoma or ocular hypertension. Adv Ther. 2014; 31:1213-1227. [Free PMC article] [PubMed] [Google Scholar] |
| [16] | Wang N., Lu D.V., Pan Yu., Astakhov Yu., Yureva T., Adevale A., Walker T.M. Comparison of the effectiveness of reducing intraocular pressure and the safety of a fixed combination of brinzolamide/brimonidine with simultaneous use of brinzolamide and brimonidine for the treatment of open-angle glaucoma or ocular hypertension. Klin Ophthalmol. 2020; 14:221-230. [Free PMC article] [PubMed] [Google Scholar] |
| [17] | Kozobolis V., Panos G.D., Konstantinidis A., Labyris G. Comparison of fixed combination therapy with dorzolamide/timolol and brinzolamide/brimonidine in the treatment of primary open-angle glaucoma. Eur J Ophthalmol. 2017; 27: 160-163. [PubMed] [Google Academy] |
| [18] | Joe H.J., Jin S.V. Comparison of various combinations of maximum drug therapy to reduce intraocular pressure in primary open-angle glaucoma: a 12-month retrospective series of consecutive cases. Jpn J Ophthalmol. 2019; 63: 322-327. [PubMed] [Google Academy] |
| [19] | Wy S, Kim YK, Jeoung JW, Park KH, Ha A. Comparison of two combinations of maximum drug therapy to reduce intraocular pressure in primary open-angle glaucoma. Korean J Ophthalmol. 2020; 34:19-26. [Free PMC article] [PubMed] [Google Scholar] |
| [20] | Moosavi R, Ansari E. Fixed brinzolamide/brimonidine combination: simplification of glaucoma treatment regimens. Ophthalmol Ter. 2018; 7: 397-403. [Free PMC article] [PubMed] [Google Scholar]. |





 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML

