-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(6): 1559-1562
doi:10.5923/j.ajmms.20241406.19
Received: May 22, 2024; Accepted: Jun. 9, 2024; Published: Jun. 14, 2024

Long-term Results of Chemoradiotherapy for Locally Advanced Cervical Cancer Considering the Histological Structure of the Tumor and PD-L1 Expression
Аlbina Rashitova
Department of Nuclear Science & Medical Radiology, Bukhara State Medical Institut named after Abu Ali ibn Sino, Bukhara, Uzbekistan
Correspondence to: Аlbina Rashitova, Department of Nuclear Science & Medical Radiology, Bukhara State Medical Institut named after Abu Ali ibn Sino, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

To determine the effectiveness of chemoradiotherapy for locally advanced cervical cancer, taking into account the histological structure and PD-L1 expression. From January 2020 to December 2023, a retrospective research was carried out at the Republican Scientific and Practical Medical Center of Oncology and Radiology (B.F RSPMCOR) branch in Bukhara. The observation findings for 112 individuals with stage IIb–IIIb cervical cancer who had chemoradiotherapy made up the study material. This study demonstrated a more malignant nature of adenogenic forms of cervical cancer, as well as an aggressive course of the disease with PD-L1 expression. Such patients exhibit early metastases, as well as unsatisfactory results of the conducted chemoradiotherapy. Adenogenic forms of cervical cancer, as well as squamous cell, with positive PD-L1 expression, should be considered as the most aggressive tumors with an unfavorable prognosis, requiring more detailed morphological study and personalized treatment.
Keywords: Cervical cancer, Chemoradiotherapy, PD-L1
Cite this paper: Аlbina Rashitova, Long-term Results of Chemoradiotherapy for Locally Advanced Cervical Cancer Considering the Histological Structure of the Tumor and PD-L1 Expression, American Journal of Medicine and Medical Sciences, Vol. 14 No. 6, 2024, pp. 1559-1562. doi: 10.5923/j.ajmms.20241406.19.
1. Introduction
- Cervical cancer ranks eighth in frequency among all oncological diseases, standing out as the most common gynecological cancer. Each year, over 660,000 new cases of this disease are registered worldwide, underscoring its global prevalence and significance in the healthcare sphere. More than 330,000 women die annually from this type of cancer, highlighting its high lethality [1]. In Uzbekistan, cervical cancer ranks second in both incidence and mortality among women. In 2022, 1,851 new cases of invasive cervical cancer were identified, with 969 cases resulting in fatal outcomes from this disease [1]. Disease progression is observed in more than 30% of patients [2]. The overall 5-year survival rate for patients with metastatic cervical cancer is approximately 17% [3], which is extremely low. This is due to the limited effectiveness of current treatment methods, which often fail to completely halt disease progression. Existing therapeutic approaches, including chemotherapy, radiotherapy, and surgical intervention, often do not provide long-term control over the metastatic process.Currently, in Uzbekistan, according to the treatment protocol for patients with stage IIb and higher cervical cancer (CC), the following specialized treatment methods are utilized: chemoradiotherapy, with radiotherapy being the predominant method used independently or in combination with other methods in over 90% of cases. Surgical treatment is typically performed in patients with early-stage disease followed by radiotherapy, and polychemotherapy is administered in cases of metastatic or recurrent disease. Radiotherapy for CC, like many other malignant tumors, remains the most important non-surgical treatment method for solid tumors, with approximately 50-60% of all cancer patients receiving radiotherapy. The inclusion of radiotherapy in treatment regimens reduces disease recurrence and improves overall survival for most common types of cancer [4,5,6]. In addition to the direct cytoreductive effect of radiotherapy, emerging evidence suggests that the generation of anti-tumor immune reactions may play a significant role in the efficacy of this treatment [7,8].Thus, identifying and blocking key regulators of immunosuppression could significantly enhance anti-tumor immune reactions and potentially improve treatment outcomes for patients. One such marker is the expression of PD-L1. The ligand of the PD-1 receptor is PD-L1, a transmembrane protein. PD-L1 inhibits the cytotoxic activity of cytotoxic lymphocytes when it binds to the PD-1 receptor on these lymphocytes, reducing their ability to destroy tumor cells. Therefore, PD-L1 blockade may be an effective strategy to overcome immunosuppression and enhance the effectiveness of anti-tumor therapy.The PD-1/PD-L1 axis is involved in maintaining peripheral tolerance and modulating acute inflammatory responses by inhibiting the function of T cells, such as loss of T-cell receptor signaling or apoptosis of activated T cells [9,10]. In addition to binding to PD-1, PD-L1 can also suppress T-cell function through interaction with CD80 [11]. Although PD-L1 is barely detectable in most normal tissues, its expression has been described in numerous malignant neoplasms [12].In this context, studying the PD-L1 status in patients with cervical cancer (CC) assumes particular significance. Undoubtedly, there is a correlation between prognosis and treatment efficacy for locally advanced CC depending on the histological subtype of the tumor and the level of PD-L1 expression. This approach may allow for more accurate prediction of treatment response and adaptation of therapeutic strategies to the individual characteristics of each patient. Exploring such parameters opens new horizons in understanding tumor biology and its interaction with the immune system. Ultimately, this may lead to the development of personalized treatment methods that are more effective and targeted, thereby increasing the chances of successful treatment outcomes and improving the quality of life for patients.Study Objective: To determine the long-term effectiveness of chemoradiotherapy for locally advanced cervical cancer (LACC) and its correlation with PD-L1 expression in tumor cells.
2. Materials and Methods
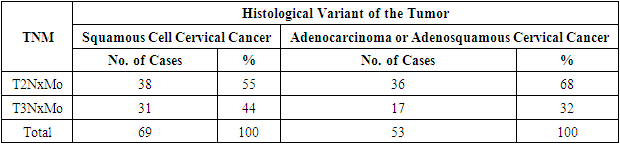
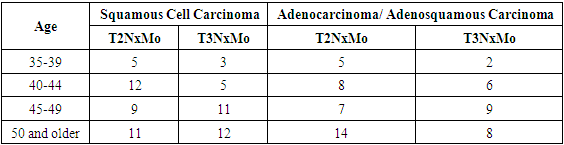
- A retrospective study was conducted at the B.F. National Cancer Research Center from January 2020 to December 2023. The study included 112 patients with stage IIb–IIIb cervical cancer who received chemoradiotherapy. Before treatment initiation, extensive clinical examination was performed, including medical history collection, complaint assessment, colposcopy, bimanual and rectovaginal examinations, review of biopsy histology, and assessment of PD-L1 expression. Patients were divided into two groups: 69 patients with squamous cell carcinoma and 53 patients with adenocarcinoma or adenosquamous carcinoma.For a more detailed analysis, each patient was classified into a specific disease stage based on the following criteria: the size and localization of the primary tumor (T), the extent of involvement of regional lymph nodes (N), and the presence of distant metastases (M). This allowed for accurate staging and distribution of patients into groups depending on the severity and spread of the disease.Table 1 provides a detailed distribution of patients according to disease stages based on the TNM system, allowing not only to assess the prevalence of different stages of cervical cancer among the study participants but also to identify correlations between disease stages and various histological tumor types.
|
|
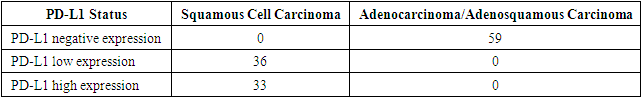
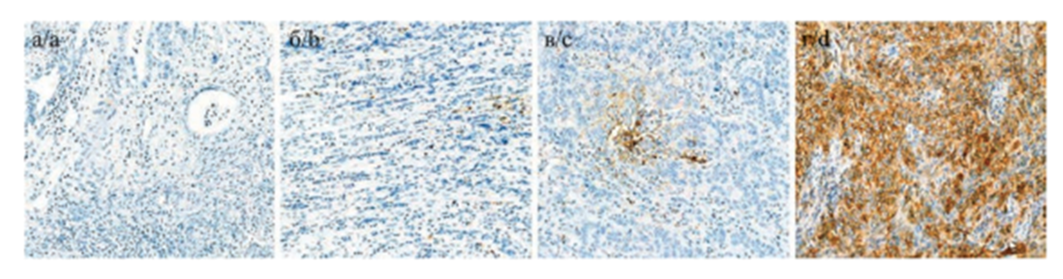 | Figure 1. Visual examples of cell staining at different levels of PD-L1 expression a, b - negative expression; c - low expression; d - high expression |
|
3. Research Results
- It is worth noting that the effectiveness of the therapy was assessed using indicators of disease-free and overall survival. In patients with squamous cell carcinoma of the cervix, the three-year survival rate was 95%, while for adenocarcinoma forms, it was lower at 88%. From these data, it follows that the survival rate of patients with squamous cell carcinoma of the cervix is significantly higher than that of those with adenocarcinoma forms.Thirteen (18.8%) patients diagnosed with squamous cell carcinoma of the cervix died. In the first year, distant metastases to the lungs and bones were detected in four patients, which subsequently led to death. Moreover, these patients had high PD-L1 expression. Generalization of the tumor process over the next 3 years led to fatal outcomes in 9 patients.Within the first year, 18 (33.1%) patients with adenocarcinoma forms of cervical cancer died, with late stages T3bN0M0. Generalization of the tumor process and distant metastases over the next 3 years led to fatal outcomes in 17 patients.This data underscore the differences in survival and causes of mortality between patients with different histological types of cervical cancer. High PD-L1 expression in patients with squamous cell carcinoma and late stages of adenocarcinoma cervical cancer are important factors influencing the prognosis and outcome of the disease.
4. Conclusions
- Thus, it can be concluded that adenocarcinoma forms of cervical cancer are the most aggressive and poorly responsive to standard treatment. PD-L1 expression was detected in every case of squamous cell carcinoma; however, the study showed that high expression (>49%) is a negative prognostic factor, with early metastasis and unsatisfactory treatment outcomes observed in this patient group. All patients with these morphological tumor characteristics should be considered as having the most aggressive tumors with an unfavorable prognosis, requiring more detailed morphological examination, specifically mandatory assessment of PD-L1 expression in all patients with confirmed squamous cell carcinoma.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML