Boltaeva Malika Miralievna
Bukhara State Medical Institute, Bukhara, Uzbekistan
Correspondence to: Boltaeva Malika Miralievna, Bukhara State Medical Institute, Bukhara, Uzbekistan.
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The article presents the results of our own research based on the results of a retrospective study of risk factors for the development of ischemic hypoxic encephalopathy in newborns.
Keywords:
Agitation syndrome, Fetal hypoxia, Respiratory failure
Cite this paper: Boltaeva Malika Miralievna, The Main Causes of Perinatal Damage to the Central Nervous System in Newborns, American Journal of Medicine and Medical Sciences, Vol. 14 No. 6, 2024, pp. 1543-1545. doi: 10.5923/j.ajmms.20241406.15.
1. Introduction
Central nervous system damage in neonatology and pediatric neurology in neonatal jackalocks is the main etiological factor in the development of the pathological process, predicting the further development of children and their individual characteristics. [1,3,9,10]. According to World Statistics, the detection of hypoxic-ischemic encephalopathy in full-born babies is 2.0-9.0 to 100, antenatal hypoxia lies as the main etiopathogenetic factor in babies and is the main cause of the development of hypoxic-ischemic encephalopathy at birth. The purpose of our retrospective analysis is to identify risk factors for the development of fetal hypoxia and the formation of ischemic-hypoxic encephalopathy in newborns, and to study its withdrawal characteristics in the neonatal period [5,7].The purpose of the study. Study of risk factors for the development of ischemic - hypoxic encephalopathy in newborns.
2. Materials and Methods of Research
We in this study analyzed the health status of 100 mothers and newborns from the history of the disease, the specifics of the course of pregnancy and the process of childbirth, methods of childbirth, data on obstetrics-gynecological and genealogical Anamnesis. The study was conducted at the Bukhara Regional Perinatal Center. Retrospectively, newborns diagnosed with” fetal growth delay syndrome “-” perinatal brain damage “and” hypoxic-ischemic encephalopathy " - included single fetuses, gestational age 20 to 35 years, with fetal hypoxia suspected at 36 weeks or older. In our study, special attention was paid to the condition of the fetus, the presence of nodules or folds in the umbilical system to determine its indicators.
3. Research Results
In the main observation groups 1 and 2, various obstetric and somatic pathologies were identified during pregnancy. 35 (34.3%) women in labor were diagnosed with various extragenital disorders. Group 1 was dominated by the following pathologies: anemia-62 (60.8%) diseases of the urinary system-40 (39.2%), diseases of the cardiovascular system-10 (9.8%), thyroid diseases-17 (16.6), diseases of the gastrointestinal tract-11 (10.7%). In Group 2, anemia occurs in 51 (50.0%) patients, in diseases of the urinary system — 32 (31.3%), in diseases of the cardiovascular system-12 (11.7%), in diseases of the thyroid gland-13 (12.7%), diseases of the gastrointestinal tract-10 (9.8%). These indicators are shown in Figure 1.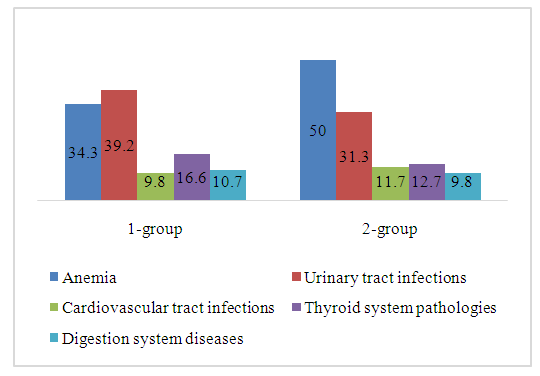 | Figure 1. The ratio of pathologies encountered by systems in both major groups, in% |
The two groups of women had Level 1 obesity in 15 (14.7%), with 7 in Group 1 (6.8%) and 8 in Group 2 (7.8%), while Level 2 obesity accounted for 11 overall (10.7%) in Group 1 and 2 respectively, with 6 in Group 1 (5.8%) and 5 (4.9%) in Group 2. Obesity in the 3rd degree was diagnosed in the 1st group with 3 (2.9%) and in the 2nd group (3.9%) with a total of 7 (6.8%). In 7 out of 10 pregnant women with increased body weight, labor activity was complicated by anomalies. It should be noted that in most cases, a combination of several diseases has been noted, which significantly complicates the process of pregnancy and childbirth. This case is shown in Figure 2.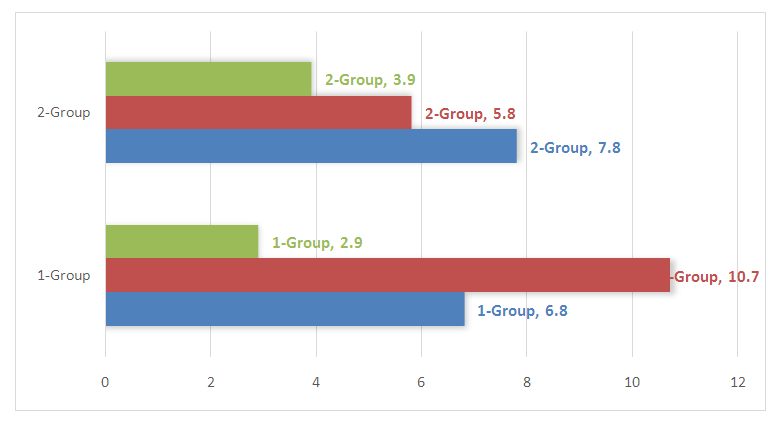 | Figure 2. The ratio of the identified obesity levels in the main group |
Chronic fetoplasental insufficiency (sfe) accounted for 92 (90.1%) of all examined pregnant women, and was distributed in groups of 52 (56.5%) to 40 (44.5%). At the same time, fetal growth retardation syndrome (HOOQS) was found to be 10 (9.8%) respectively, compared to 6 (5.8%) and 4 (3.9%) in groups. In addition, UTT (ultrasound examination) found that 12 patients in Group 1 (11.7%) and 10 in Group 2 (9.3%) had low water in the third trimester of pregnancy, with the exception of polyhydroamniosis in Group 1, 10 (9.8%) and 9 (8.8%) were found in pregnancies.In patients, umbilical cord wrapping as a result of UTT was 21.3% overall. In this case, this situation was distributed in both groups in a ratio of 11.3% and 10%. This condition causes fetal hypoxia to deepen again. The analysis did not reveal statistically significant differences between the groups. In patients, umbilical cord wrapping as a result of UTT was 21.3% overall. In this case, this situation was distributed in both groups in a ratio of 11.3% and 10%. This condition causes fetal hypoxia to deepen again. The analysis did not reveal statistically significant differences between the groups.Dopplerometry performed in patients revealed a slow circulation in the right artery of the placenta, the uterus. It is also indicated by the fact that the rate of mining leakage in the fetal midbrain artery also increases relative to the norm indicators of resistance.A vacuum extractor was used in Group 1, 12 (11.7%), and Group 2, 10 (9.8%), due to worsening fetal condition. Caesarean section surgery was performed in Group 1, with 15 cases (14.7%) and Group 2 in 18 cases (17.6%).
4. Conclusions
When using the combined method of fetal hypoxia diagnosis, acute fetal hypoxia is significantly less diagnosed (p<0.05) - 21.0 and 35.2% in Group 1 and 20% in Group 2, respectively, with impaired First-Order fetoplasental complex.
References
| [1] | Shellhaas RA, Ng CM, Dillon CH, Barks JD, Bhatt-Mehta V. (2013). Population pharmacokinetics of phenobarbital in infants with neonatal encephalopathy treated with therape. |
| [2] | Medical hypothermia in the treatment of severe asphyxiation at birth [Primenenie lechebnoy gipotermii pri lechenii tyazheloy intranatal’noy asfiksii]. Universum: Meditsina i farmakologiya: elektronnyy nauchnyy zhurnal, (11). Available at: http://7universum.com/ru/med/archive/item/2714 (date of the access 10.01.2016). |
| [3] | Thoresen M, Tooley J, Liu X, Jary S, Fleming P, Luyt K, Jain A, Cairns P, Harding D, Sabir H. (2013). Time is brain: starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology, 104, 228-233. |
| [4] | Traudt CM, McPherson RJ, Bauer LA. (2013). Concurrent erythropoietin and hypothermia treatment improve outcomes in a term nonhuman primate model of perinatal asphyxia. Dev. Neurosci., 35, 491-503. |
| [5] | Sabir H, Jary S, Tooley J, Liu X, Thoresen M. (2012). Increased inspired oxygen in the first hours of life is associated with adverse outcome in newborns treated for perinatal asphyxia with therapeutic hypothermia. J. Pediatr., 161 (3), 409-416. |
| [6] | Boltaeva M.M., Negmatullaeva M.N. – Diagnostic criteria for fetal hypoxia // New Day in Medicine 2023 8(58): 2-8. |
| [7] | Vohr BR, Stephens BE, McDonald SA. (2013). Cerebral palsy and growth failure at 6 to 7 years. Pediatrics, 132, 905-914. |
| [8] | TOBY study group. Predictive value of the amplitude integrated EEG in infants with hypoxic ischaemic encephalopathy: data from a randomized trial of therapeutic hypothermia. Arch. Dis. Child Fetal Neonatal Ed., 99, 80-82. 12. AzzopardiD, Strohm B, Marlow N. (2014). |
| [9] | Boltaeva Malika Miralievna. Modern aspects of forecasting and early diagnosis of fetal hypoxia to reduce the perinatal incidence of hypoxic- ischemic genesis, // Web of scientisit: International Scientific research journal 2023; 4(4): 959-963. |
| [10] | Shankaran S, Pappas A, Scott A, McDonald SA, Vohr BR, Hintz SR, Epi MS, Yolton K, Gustafson KE, Theresa M. (2012). Childhood outcomes after hypothermia for neonatal encephalopathy. N. Engl. J. Med., 36, 2085-2092. |
| [11] | Natarajan G, Shankaran S, Laptook AR, Pappas A, Bann CM, McDonald SA, Das R, Higgins D, Hintz SR, Vohr BR. (2013). Apgar scores at 10 min and outcomes at 6–7 years following hypoxic-ischaemic encephalopathy. Arch. Dis. Child Fetal Neonatal Ed., 98, 473-479. |
| [12] | Chakkarapani E, Dingley J, AquilinaK, Osredkar D, Liu X, Thoresen M. (2013). Effects of xenon and hypothermia on cerebrovascular pressure reactivity in newborn global hypoxic-ischemic pig model. J. Cereb. Blood Flow Metab., 33, 1752-1760. |




 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML