-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(6): 1518-1521
doi:10.5923/j.ajmms.20241406.09
Received: May 15, 2024; Accepted: Jun. 11, 2024; Published: Jun. 13, 2024

Morphology of the Aorta in Metabolic Syndrome
B. L. Khoshimov1, S. M. Akhmedova2
1Doctor of Philosophy (PhD) in Medical Sciences, Alfraganus University, Tashkent, Uzbekistan
2Doctor of Medical Sciences (DSc), Professor, Tashkent Medical Academy, Tashkent, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Studying the nature and initial changes of the morphological changes observed as a result of hypokinesia and the consequences of metabolic syndrome is an urgent medical problem. A high–calorie diet throughout life is considered one of the main causes of metabolic syndrome. In understanding the pathogenesis of metabolic syndrome and hypodynamia, it is important to know the morphological changes observed in these pathologies. As an object of research, we studied the morphological changes in arteries of elastic type. The results revealed destructive and defragmentation changes in fibrous structures in the walls of elastic-type arteries.
Keywords: Morphological changes, Aorta, Metabolic syndrome, Elastic vessels, Hypokinesia, High–calorie diet, Hypodynamia, Destructive changes, Fibrous structures, Elastic type, Walls
Cite this paper: B. L. Khoshimov, S. M. Akhmedova, Morphology of the Aorta in Metabolic Syndrome, American Journal of Medicine and Medical Sciences, Vol. 14 No. 6, 2024, pp. 1518-1521. doi: 10.5923/j.ajmms.20241406.09.
Article Outline
1. Introduction
- In recent years, hypokinesia is become not only a medical but also a social problem. Several factors cause hypokinesia: high automation of production, transition to a sedentary lifestyle, and being in bed after certain diseases. The classification of hypokinesia is presented in several literature. According to the World Health Organization, about 60% of the world’s population does not have enough physical activity to lead a healthy lifestyle [1,8]. As a result of a lifestyle caused by inactivity, 1.9 million deaths are observed in the literature. Today, many sources provide information about the morphofunctional changes that occur in the body under the influence of the negative consequences of hypokinesia. As a result of inactivity, negative consequences develop in all organs and systems. The researches of several scientists show that as a result of hypokinesia, not only muscles but also changes in mineral saturation of bone tissue develop [6,9]. In recent years, information has been received about the development of dystrophy and disintegration in spongy and flat bones in the locomotor system under conditions of hypokinesia. In the case of inactivity, changes such as negative changes in the respiratory system, impaired liver function, and decreased reproductive organs have been observed. In the conditions of hypokinesia, a violation of carbohydrate metabolism, a change in the water-salt balance has been determined [2,3,5]. These metabolic changes involve many unsolved problems involving the same complex mechanisms in pathogenesis [4,7,10]. When the diameter of blood vessels decreases, the speed of blood flow also decreases. It is estimated that 10% to 15% of the total blood volume is in the arterial circulatory system. This feature of high systemic pressure and low volume is characteristic of the arterial system. There are two main types of arteries in the body: elastic arteries and muscular arteries. Muscular–type arteries include those with anatomical names such as the brachial artery, the brachial artery, and the femoral artery. Muscular–type arteries have more smooth muscle cells in the interstitial layer than elastic arteries. Elastic-type arteries are the arteries closest to the heart (aorta and pulmonary arteries), and they contain more elastic tissue in their interstitial layer than muscular-type arteries. This feature of elastic-type arteries allows maintaining a constant pressure gradient against them, despite the effect of the heart’s constant contraction (pumping).The purpose of the research work is to study the thoracic aorta's morphological and morphometric changes in experimental hypodynamia and metabolic syndrome.
2. Research Material and Methods
- Mature white laboratory rats weighing 180–200 grams were used as study material. The white rats taken for the experiment were divided into 2 groups. For morphological studies, the elastic type of the thoracic aorta was taken, and the histological sections prepared on a rotor microtome with a thickness of 8–10 microns were stained with hematoxylin and eosin, Van Gison, and Weigert methods. The first group was the control group, 10 rats without clinical signs of somatic and infectious diseases were taken. Rats in the control group were continuously fed a conventional diet with free food and water ad libitum. In our second group, a total of 45 rats were used to induce the experimental metabolic syndrome model. To create a model of hypodynamism, special cage pens were used. In this case, the area of the cages was kept at no less than 150 cm². After denying the signs of infectious and somatic diseases, healthy rats were placed in a special cage, and fed a diet rich in fat and carbohydrates. The diet of rats is 60% laboratory feed, 20% sheep fat, 20% fructose. A 20% solution of fructose was given instead of drinking water. Rats with hypodynamia and metabolic syndrome formed the experimental group and were euthanized 30, 60, and 90 days after the experiment. For morphological studies, the thoracic aorta was taken, and the histological sections prepared on a rotor microtome with a thickness of 8–10 microns were stained with hematoxylin and eosin, Van Gion, and Weigert methods. In histological preparations, the structures of the cytoplasm of the blood vessel wall, and the state of the collagen elastic fibres were determined. The thickness of the wall of blood vessels, the thickness of the intima, and the separate media were calculated by the morphometric method.Laboratory white rats in the control and experimental groups were kept in the same conditions of the vivarium. G.G. Avtandilov method and NanoZoomer (REF C13140–21.S/N000198/HAMAMATSU PHOTONICS/431–. 3196 JAPAN), Hamamatsu (QuPath–0.4.0, NanoZoomer Digital Pathology Image) morphometric computer program are used for conducting morphometric examinations. The obtained data were determined in the statistical section of Microsoft Excel 2010, the arithmetic means Mni, the average error m of the relative sizes and the accuracy coefficient t. Photomicrographs of histological preparations were captured using a CX40 model OD400 camera microscope.
3. Results and Discussions
- The analysis of the obtained data showed that the thoracic aorta belongs to the type of arteries of elastic type. The basis of the thoracic aorta is an elastic skeleton. In the cross-section of the thoracic aorta, 3 parts of its wall can be seen: inner, middle and outer walls (Figure 1). The inner layer of the thoracic aorta is composed of inner endothelial cells, and a subendothelial layer consisting of connective tissue fibres touching it. It can be seen that the nuclei of endotheliocytes are oval and are located at different distances from each other. It can be seen that the endotheliocytes are oriented perpendicular to the axis of the blood vessel wall (Figure 2).
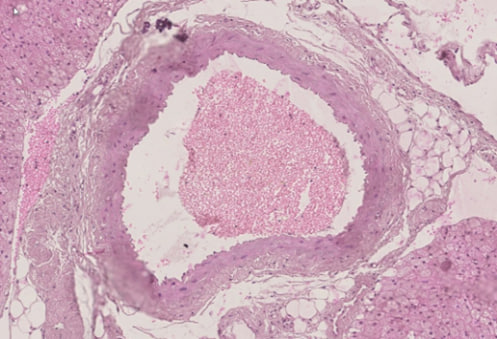 | Figure 1. Thoracic aorta. In the cross–section of the thoracic aorta, 3 parts of its wall can be seen: inner, middle and outer walls. Paint G.E. The size is 10×10 |
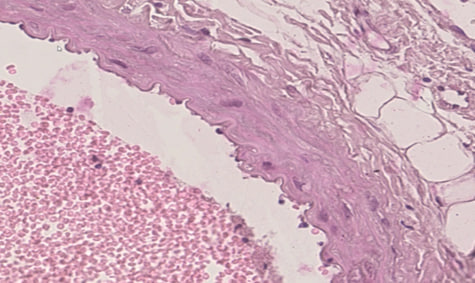 | Figure 2. The inner layer of the thoracic aorta is composed of inner endothelial cells, and a subendothelial layer consisting of connective tissue fibres touching it. Paint G.E. The size is 20×10 |
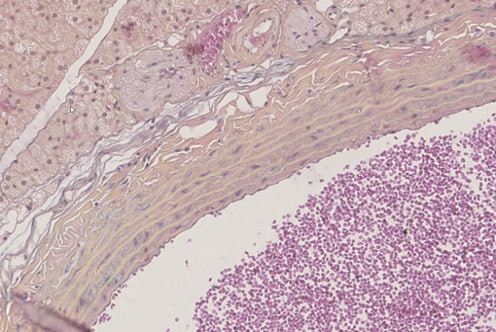 | Figure 3. In the middle layer, it can be seen that it consists of several rows of elastic membranes and smooth muscle fibres between them. Paint G.E. The size is 20×10 |
 | Figure 4. The middle layer of elevated liposclerosis (1) is uniformly muscular and fibrous, with interstitial edema (2). Paint G.E. The size is 20×10 |
 | Figure 5 |
4. Conclusions
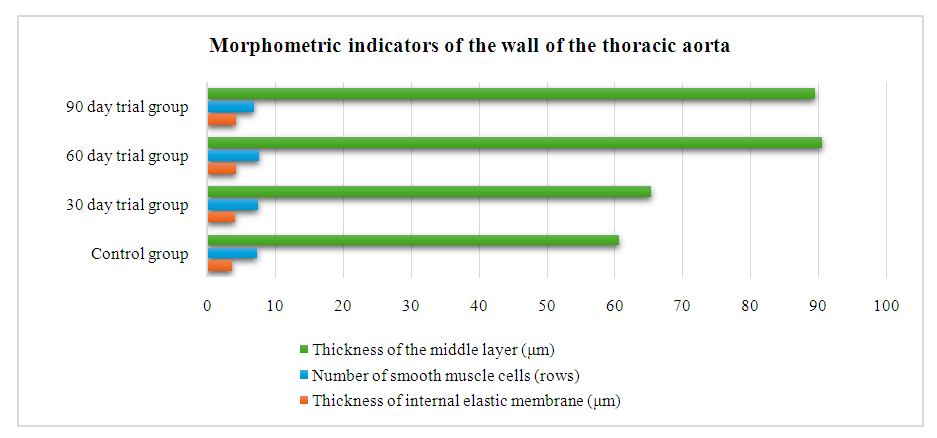
- In experimental hypokinesia and metabolic syndrome, there is a thickening of the internal elastic membrane of the wall of the thoracic aorta, a decrease in the thickness of the middle layer, and a decrease in the number of smooth muscle cells in the vascular wall.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML