-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(6): 1483-1488
doi:10.5923/j.ajmms.20241406.02
Received: May 15, 2024; Accepted: Jun. 1, 2024; Published: Jun. 7, 2024

The Role of Inflammatory Cytokines and the Effect of Complex Treatments on Changes in Vitamin D and Parathyroid Hormone Levels in Chronic Heart Failure
Abdigaffar G. Gadaev1, Shovkat Sh. Quchkorov2
1Researcher, Tashkent Medical Academy, Uzbekistan
2Researcher, Fergana Public Health Medical Institute, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The article examines the association of inflammatory cytokines with vitamin D and parathyroid hormone levels in patients with chronic heart failure. Also, on the basis of standard treatment, the combined use of sodium glucose type 2 inhibitor empagliflozin and vitamin D has been found to have a positive effect on inflammatory cytokines, vitamin D, and parathyroid hormone indicators.
Keywords: Ischemic heart disease (IHD), Chronic heart failure (CHF), Endocrine, Inflammatory, Hypovitaminosis D, Hypovitaminosis D, Calcium, Phosphorus microelements
Cite this paper: Abdigaffar G. Gadaev, Shovkat Sh. Quchkorov, The Role of Inflammatory Cytokines and the Effect of Complex Treatments on Changes in Vitamin D and Parathyroid Hormone Levels in Chronic Heart Failure, American Journal of Medicine and Medical Sciences, Vol. 14 No. 6, 2024, pp. 1483-1488. doi: 10.5923/j.ajmms.20241406.02.
Article Outline
1. The Urgency of the Problem
- Chronic heart failure (CHF) is a complex of multifactorial and dangerous syndromes, characterized by high morbidity and mortality, a sharp decrease in patients’ functional status and quality of life, as well as extremely high costs of treatment. According to data, more than 64 million people in the world have this disease. Therefore, activities aimed at reducing the incidence of CHF are global and one of the priority directions. At the same time, although there is a tendency to its decrease in economically developed countries, the introduction of modern treatment methods for ischemic heart disease (IHD), the improvement of patients’ quality of life and its duration, the increase of elderly people among the population, and ultimately the number of patients with IHD causing the number to increase. In population studies, its prevalence among the population is 1-2%, with an increase in age of more than 10% [4,8,14,23].It is known that CHF is a disease with endocrine, inflammatory and metabolic disorders and polyorgan damage [17]. Disturbances in the metabolism of calcium, phosphorus microelements and related parathormone and vitamin D are also observed in CHF. As a result of this disease, compensatory mechanisms are developed, and the increase in phosphate causes an increase in fibroblast growth factor-23 (FGF-23), a decrease in vitamin D, and an increase in parathyroid hormone [5,6]. Vitamin D deficiency activates the renin-angiotensin-aldosterone system, causing arterial hypertension and inflammation [12,13].An epidemiological study conducted in the USA showed that vitamin D plays an important role in the normal functioning of the cardiovascular system. In particular, there is a proportional increase of IHD in hypovitaminosis D, arterial hypertension, diabetes mellitus to the equatorial distance. It has been confirmed that the prevalence of vitamin D deficiency and death from cardiovascular diseases is high in the winter months, i.e. in the days when the activity of the sun’s rays is reduced [20].About 1 billion people in the world have vitamin D deficiency (<20 ng/ml) or deficiency (<21-29 ng/ml). According to the 2018 US National Health and Nutrition Examination Survey (NHANES), the prevalence of this vitamin deficiency was 28.9% [16].In CHF, hypoxic processes primarily stimulate the production of hypoxia-inducible factor 1 and α-tumor necrosis factor (α-TNF) in cardiomyocytes, leading to the activation of monocytes and macrophages [7]. Their activation increases the synthesis of a number of inflammatory cytokines and causes deeper damage to the myocardial cells that have been exposed to ischemia [3].Therefore, activation of the immune system and systemic inflammatory processes play an important role in the progression and development of the disease in patients. Regardless of its etiology, serum levels of pro-inflammatory cytokines have been found to be significantly higher than normal in CHF [18,24].Angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists, ß-blockers, mineralocorticoid receptor antagonists have been used for many years in the treatment of CHF. In recent years, drugs such as sacubutril-valsartan and glucose sodium cotransporter type 2 inhibitors (GSTI-2i) have also been included in the standard treatment of CHF [14].CHF found that the use of glucose-sodium cotransporter 2 inhibitors in patients with low left ventricular ejection fraction (LVEF) is highly effective. Currently, dapagliflozin, empagliflozin, canagliflozin and other drugs belonging to this group have been created [21,22]. However, despite the positive results achieved in the treatment of CHF in recent years, the death rate from it is still high. In most cases, it is a comorbidity of the disease, and among them, GFR is one of the leading causes of death. There is insufficient evidence that vitamin D deficiency is associated with CHF and its adverse outcomes. However, it is still controversial whether adding it to treatments can reduce cardiovascular disease and improve the outcome of CHF [11]. According to Witham and co-authors, the addition of vitamin D to the treatment did not have a positive effect on the functional status and quality of life of elderly patients with CHF [19]. In contrast, Shedeed and co-authors found that vitamin D administration in youth with CHF resulted in significant positive changes in heart function and a reduction in inflammatory markers [15].Inflammatory mediators are known to play a critical role in the pathogenesis of ventricular remodeling, and CHF is evidence-based as a serum biomarker of severity and prognosis [9,10]. Several studies have shown that circulating parathyroid hormone CHF is directly related to weight and may serve as a biomarker of weight [1,2].The pooled results of a meta-analysis by Jiang WL and co-authors found that adding vitamin D to the treatment of patients with CHF resulted in significant reductions in tumor necrosis factor-alpha, C-reactive protein, and parathyroid hormone. Therefore, they concluded that the recommendation of this vitamin in CHF reduces inflammatory factors and parahormone through a protective function [11].But until now, the effect of CHF when used together with vitamin D, ß-blockers, mineralocorticoid receptor antagonists, sacubitril/valsatan, angiotensin-converting enzyme inhibitors and sodium glucose cotransporter type 2 inhibitors included in their complex has not been covered in the literature.
2. Materials and Methods
- 120 CHF Ⅱ and Ⅲ FC patients with advanced renal dysfunction were included in the study. In them, serum creatinine and glomerular filtration rate (GFR), which is a traditional test method for evaluating kidney dysfunction, were taken as criteria. Patients included in the follow-up were divided into two main and control groups according to the treatment received at the beginning. The main group consisted of 80 patients, and their average age was 66.5±5.7, men 43 (53.75%) - women 37 (46.25%). Among them, patients with CHF Ⅱ and Ⅲ FC were 14 (17.5%) and 66 (82.5%), respectively. GER in the main group was equal to 80.6 ± 5.5 ml per 1 minute per 1.73 m2 of body surface.The control group consisted of 40 patients with a mean age of 67.6 ± 5.5 ha, 20 (50%) men and 20 (50%) women, including 8 (20%) patients with CHF Ⅱ and Ⅲ FC, respectively. and made up 32 (80%). CHF in the main group was equal to 78.4 ± 5.2 ml per 1 minute per 1.73 m2 body surface.The main and control group of patients involved in the study were divided into two subgroups based on the serum vitamin D levels during the examinations. The first subgroup was made up of patients whose blood serum vitamin D level decreased from normal values (Vit D≤30, ng/ml) and the second subgroup was made up of patients whose level was maintained (Vit D≥30, ng/ml). 40% (32) of patients in the main group and 42.5% (17) of the control group were found to have reduced vitamin D levels. Patients with vitamin D deficiency in the main group were prescribed CHF complex standard treatment (sacabutril-valsartan, ß-blocker, mineralocorticoid receptor antagonist-eplerenone, sodium glucose cotransporter type 2 inhibitor-empagliflozin) and vitamin D 4000 units per day for 8 weeks. A maintenance dose of 2,000 units was then recommended for 4 weeks. Only complex standard (sacabutril-valsartan, ß-blocker, mineralocorticoid receptor antagonist-eplerenone, sodium glucose cotransporter type 2 inhibitors-empagliflozin) treatment was applied to patients with normal vitamin D levels. At this point, we would like to point out that there is no information published in the available literature about the effectiveness of sodium glucose cotransporter type 2 inhibitors and vitamin D when used in combination with GFR patients on the basis of CHF.Patients with reduced vitamin D serum levels in the control group were prescribed CHF complex standard treatment (sacabutril-valsartan, ß-blocker, mineralocorticoid receptor antagonist-eplerenone) and vitamin D 4000 units per day for 8 weeks. A maintenance dose of 2,000 units was then recommended for 4 weeks. Patients with normal vitamin D levels were prescribed only complex standard treatment (sacabutril-valsartan, ß-blocker, mineralocorticoid receptor antagonist-eplerenone).Vitamin D, parathyroid hormone, S-reactive protein, interleukin-6, and tumor necrosis factor-alpha indicators were determined in the blood serum of all subjects involved in the study, along with routine laboratory tests, before and 6 months after treatment. Intracardiac hemodynamics were assessed using echocardiography and electrocardiography, and renal functional status was assessed by calculating GFR using creatinine.
3. Research Results and Discussion
- Based on CHF, it has been proven in a number of scientific studies that in patients with developed kidney dysfunction, changes in other indicators are observed, depending on the decrease of vitamin D, calcium, on the contrary, the increase of phosphorus and parathormone. We also compared vitamin D and parathormone levels in the blood serum after the treatment in the follow-up patients. Figure 1 below shows the dynamics of vitamin D and parathyroid hormone levels in the main group of patients.
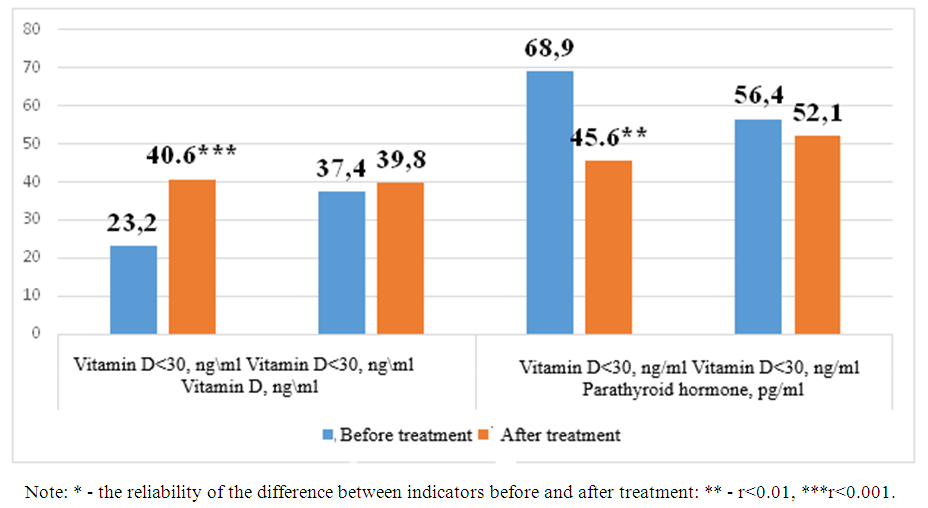 | Figure 1. Post-treatment changes in vitamin D and parathormone levels in the main observation group of patients |
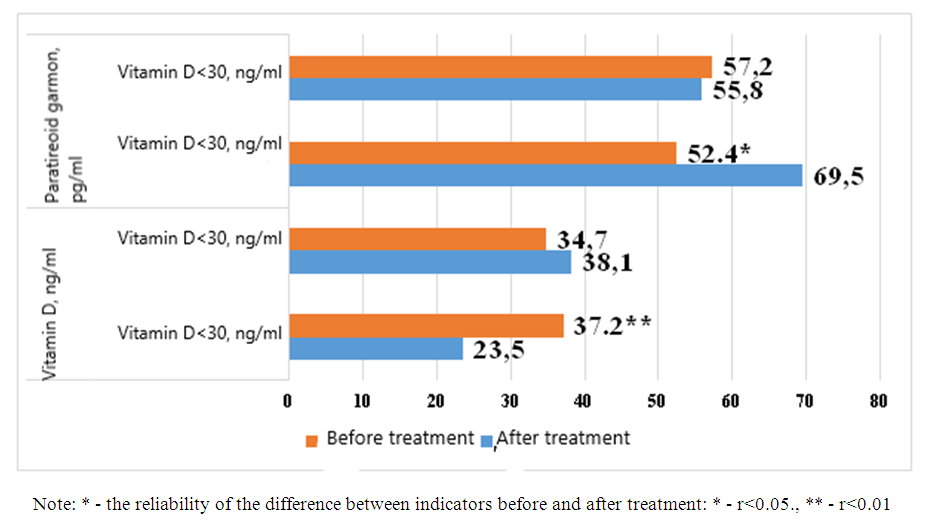 | Figure 2. Post-treatment changes in vitamin D and parathyroid hormone levels in control group patients |
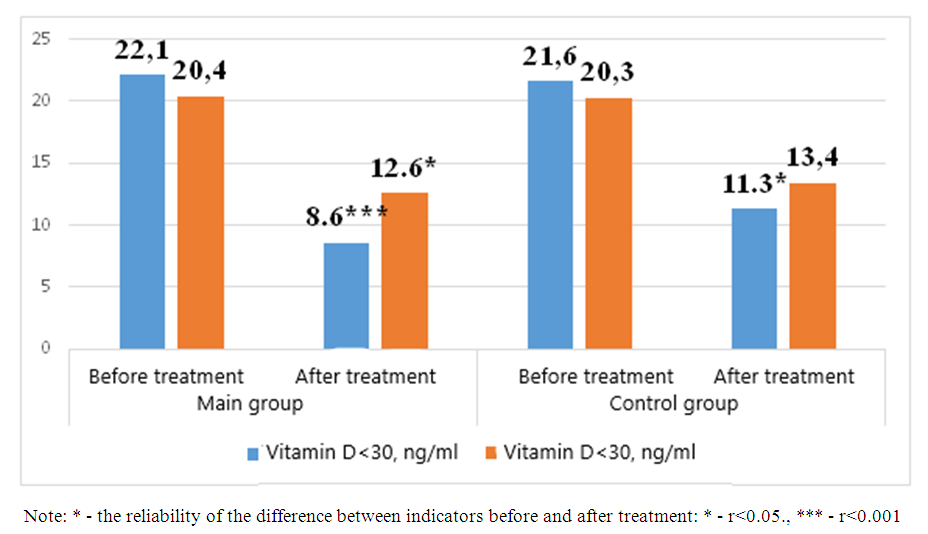 | Figure 3. Interleukin-6 levels after treatment in patients enrolled in the study |
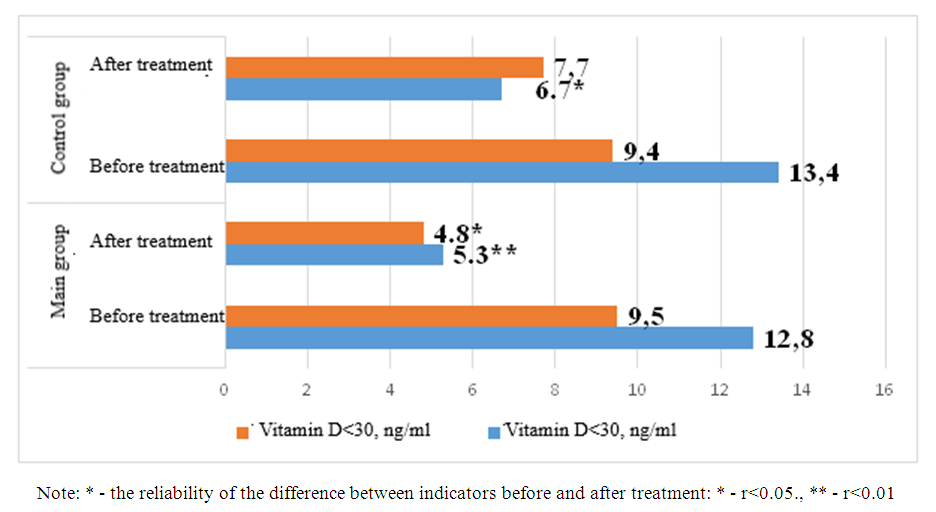 | Figure 4. Comparative analysis of post-treatment tumor necrosis factor-α indicators in study patients |
4. Conclusions
- In patients with chronic heart failure and advanced renal dysfunction, determination of vitamin D indicators in blood serum and coordination of medical treatment using it slows down the development of pathological processes in the kidneys and leads to improvement of its function. This is confirmed by the results obtained by co-prescribing empagliflozin with vitamin D in patients in the main and control groups in our study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML