-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(5): 1451-1466
doi:10.5923/j.ajmms.20241405.68
Received: Apr. 19, 2024; Accepted: May 17, 2024; Published: May 30, 2024

Assessing the Effectiveness of Topical Treatments for Recurrent Aphthous Stomatitis: A Network Meta-Analysis
Yakubova Farida Khaldarovna, Shukurova Gulnora Raxmanovna
Associate Professor at Tashkent State Pediatric Institute, Uzbekistan
Correspondence to: Yakubova Farida Khaldarovna, Associate Professor at Tashkent State Pediatric Institute, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background and Objectives: The aim was to compare the effectiveness and safety of various topical interventions for recurrent aphthous stomatitis. Methods: Following the PRISMA guidelines, this network meta-analysis examined randomized controlled trials retrieved from PubMed, Web of Science (WOS), Cochrane Central Register of Controlled Trials, and Embase. Quality assessment was conducted using Cochrane Handbook criteria. Data on healing, size reduction, symptom alleviation, recurrence, and safety were independently extracted by two authors. Network meta-analysis was performed using ADDIS and RevMan. Results: 72 trials (5272 subjects) involving 29 topical treatments were analyzed. Honey, insulin liposome gel, laser, amlexanox, glycyrrhiza, and triamcinolone showed superior efficacy. Probiotics and chlorhexidine extended ulcer intervals and reduced recurrence. Doxycycline and penicillin carried high adverse event risks. Hematologic evaluation showed no bias. Laser was ranked high for short-term size and symptom reduction, while probiotics showed long-term benefits. Conclusion: Laser is recommended for short-term intervention during exacerbation, and probiotics for long-term management during both exacerbation and remission phases of recurrent aphthous stomatitis.
Keywords: Recurrent aphthous stomatitis, Network meta-analysis, Topical intervention
Cite this paper: Yakubova Farida Khaldarovna, Shukurova Gulnora Raxmanovna, Assessing the Effectiveness of Topical Treatments for Recurrent Aphthous Stomatitis: A Network Meta-Analysis, American Journal of Medicine and Medical Sciences, Vol. 14 No. 5, 2024, pp. 1451-1466. doi: 10.5923/j.ajmms.20241405.68.
1. Introduction
- Recurrent aphthous ulcer (RAU), also known as recurrent aphthous stomatitis (RAS), is a prevalent condition affecting the oral mucosa. Its prevalence varies widely, ranging from 1.4% to 21.4% [1,2,3,4,5] as indicated by retrospective population-based studies across different countries and regions. Extensive research has been conducted on the etiology and pathogenesis of RAS. There is speculation that the oral microbiota [6], including organisms like Streptococcus [7], Helicobacter pylori [8], cytomegalovirus [9], and various other microorganisms [10], may play significant roles in ulcer formation. Additionally, systemic diseases can manifest with ulcers as a prominent phenotype [11], further complicating etiological investigations.Despite extensive research, the precise etiology and pathogenesis of RAS remain incompletely understood. Consequently, specific treatments for RAU have yet to be identified in clinical and basic trials [12]. Presently, treatment for RAS primarily focuses on symptom management, aiming to alleviate pain, facilitate lesion healing, and prolong the interval between episodes. Topical treatments, including medication, laser therapy, cryotherapy, and cautery, are considered effective for managing minor recurrent aphthous ulcers (MiRAU) and as adjuncts for major recurrent aphthous ulcers (MaRAU) [13]. Topical glucocorticoids, such as dexamethasone and triamcinolone, are commonly prescribed as first-line agents due to their anti-inflammatory and immunosuppressive properties [14]. Tetracyclines and derivatives, particularly doxycycline, are believed to inhibit ulcer formation and tissue destruction by targeting matrix metalloproteinases in the inflammatory pathway [15]. Amlexanox, recognized for its anti-inflammatory and anti-allergic effects, has gained attention in recent studies [16].Emerging therapeutic modalities, including biological and laser treatments, hold promise for managing mucosal diseases like RAS [17]. Systematic analyses have underscored the role of oral flora alterations in RAS progression [18], paving the way for topical probiotic interventions. Laser therapy has shown efficacy in accelerating tissue repair and pain relief, while traditional treatments like freezing [19] and cautery [20] remain prevalent for their beneficial effects on cell metabolism and tissue regeneration. Low serum zinc levels have been identified as a risk factor for RAS [21], prompting the recommendation for topical zinc supplementation. Moreover, natural extracts such as curcumin [22], glycyrrhiza [23], honey [24], quercetin [25], chitosan [26], aloe [27], berberine gelatin [28], diosmectite [29], allicin [30], and others have shown promise as potential topical interventions for ulcers.RAS presents as a self-limiting condition characterized by varying durations and manifestations among individuals. While most cases of recurrent aphthous stomatitis resolve within a few days, they can significantly impact daily life due to localized mucosal lesions causing discomfort, pain from chemical-mechanical irritation, and recurrent episodes [31]. Traditional meta-analyses by various authors have primarily focused on comparing the efficacy of local interventions for RAS against ineffective placebos [32,33,34]. However, these approaches limit the comparison to pairwise estimates of effect, preventing simultaneous assessment of the relative efficacy of multiple interventions.The question of which local intervention is most effective for RAS remains contentious, with a dearth of robust research evidence. Despite extensive literature searches, we have not identified a comprehensive systematic evaluation and ranking of multiple local interventions for RAS treatment. Hence, we conducted a systematic review, incorporating numerous randomized controlled trials (RCTs), to assess the efficacy and safety of up to 29 local interventions for RAS treatment. Our objective was to furnish a dependable reference for clinicians in selecting more effective topical treatment options for RAS patients.
2. Materials and Methods
- Assertion Network meta-analysis (NMA) represents a novel, high-quality analytical approach founded on the principles of homogeneity, transferability, and consistency. This method facilitates simultaneous comparisons among multiple interventions and offers a potential ranking of their effectiveness [35]. Consequently, NMA has become a cornerstone in numerous studies, enabling the provision of more robust evidence and aiding in the selection of optimal solutions. Our study adhered rigorously to the criteria and protocols governing NMA [36]. We followed relevant guidelines and standards throughout the study, ensuring compliance with software requirements, including Addis, Revman, Endnote, and others. Furthermore, our study was registered in the PROSPERO International Prospective Register of Systematic Reviews before implementation, in accordance with established guidelines (registration number: CRD42021251154). Data Sources and Search Strategy We conducted a comprehensive search across four databases—PubMed, Web of Science (WOS), Cochrane Central Register of Controlled Trials, and Embase—during the study period. The search encompassed literature published from the inception of each database up to October 1, 2021. Medical terms such as "Stomatitis, Aphthous," "Oral Ulcer," and "Clobetasol" were employed as primary search terms. Additionally, synonyms and abbreviations such as "Canker sore," "Corticosteroid," and "LLLT*" were included as keywords to broaden the search scope. The search process was independently executed by two individuals, and the Endnote literature management software facilitated the organization of search results. Further details regarding the search strategy are outlined in Tables S1–S5.Selection Criteria The randomized controlled trials (RCTs) included in this study adhered to the following criteria: (1) Confirmation of recurrent aphthous ulcers through clinical or histopathological examination, with visible ulcer-like lesions observed on any area of the oral mucosa. (2) Inclusion of simple ulcerative lesions of undetermined origin, excluding oral manifestations of systemic diseases like leukoaraiosis or diabetes mellitus, as well as specific ulcerative lesions resulting from trauma or radiotherapy. (3) Participants received only local interventions or placebos during the trial, without undergoing any other treatments that could potentially affect recurrent aphthous stomatitis (RAS) prior to or during the trial, such as systemic steroids or immunosuppressants. (4) For studies involving patients with multiple oral mucosal diseases, data related specifically to RAS were extracted. If extraction was not feasible, the study was excluded from analysis.Outcomes In this study, clinical efficacy and safety were chosen as primary outcome measures. Clinical efficacy was evaluated based on healing efficacy, size reduction effect, and symptom reduction effect. Healing efficacy was assessed by the time taken for complete healing, measured from the enrollment in the local intervention to the total resolution of ulcer-like lesions. The size reduction effect was determined using the efficacy index (EI), calculated as the ratio of ulcer reduction area to baseline ulcer area. The cumulative reduction in ulcer size across various examination days during the trial was recorded. Symptom reduction effect was also assessed using the efficacy index (EI), calculated as the ratio of reduction in pain score to baseline ulcer pain score. Individual subjects' pain levels were measured using visual analog scale (VAS) or decile scale scores on different examination days, and cumulative pain relief was calculated accordingly (VAS score: 10 cm horizontal line, marked 0 = no pain to 10 = worst pain; Decile scale: 0 for no pain, 10 for most pain). Safety evaluation included monitoring adverse events and assessing blood levels of the intervention drug. Additionally, the study extracted data related to the impact on the recurrence of RAS.Data Collection and Risk of Bias Assessment Two researchers independently conducted literature screening against predefined inclusion and exclusion criteria, assessed literature quality according to established criteria, and extracted relevant information. This process was carried out independently by the researchers and subsequently cross-verified, with any discrepancies resolved through consultation with a third party or discussion among the research team. Extracted information included details such as the first author of the included studies, publication time, country of origin, sample size, demographics (gender and age), interventions, and outcome indicators.The quality of the included studies was evaluated using RevMan 5.3 software provided by the Cochrane Collaboration. This evaluation encompassed criteria such as random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, selective reporting, and other potential biases.Furthermore, potential publication bias within the included studies was examined utilizing a funnel plotting approach.Data Synthesis and Statistical Analysis Traditional meta-analysis was conducted using RevMan 5.3 and ADDIS 1.16. Risk difference (RD) served as the efficacy indicator for dichotomous variables, while mean difference (MD) was employed for continuous variables. Each effect size estimate was accompanied by a 95% confidence interval (95% CI). Heterogeneity of test results was quantified using the I2 statistic. Heterogeneity was deemed small if p ≥ 0.1 and I2 ≤ 50%, warranting the use of a fixed-effects model for combining. Conversely, if p < 0.1 and I2 > 50%, indicating significant heterogeneity, a random-effects model was applied for combining, with sensitivity analysis conducted through subgroup analysis or individual literature exclusion.A random-effects network within a Bayesian framework model was established using ADDIS 1.16 [37]. Networks were tailored to different outcome indicators to accommodate a wide array of local interventions. Direct and indirect comparisons between interventions were made to ensure comprehensive and complete results. Statistical significance was considered at p < 0.05. ADDIS 1.16 also estimated ranking probabilities for interventions. MD for each local intervention was compared to an arbitrary control, with the convergence of the model evaluated through the number of Markov chain iterations. Variance calculations and node splitting analyses were performed to assess inconsistency in the network meta-analysis. Results were deemed inconsistent if the random effects variance significantly deviated from the inconsistency or if the discrepancy between direct and indirect evidence was deemed significant (p < 0.05).
3. Results
- Study Selection The initial database search yielded 11,962 records. Following the removal of 2388 duplicate articles, the titles and abstracts of 9574 articles were screened. Among these, 9314 articles were excluded as they did not meet the inclusion criteria. A full-text review was conducted for 260 articles, of which 186 were subsequently excluded. Ultimately, 72 eligible studies were included for qualitative and quantitative analysis (see Figure 1).
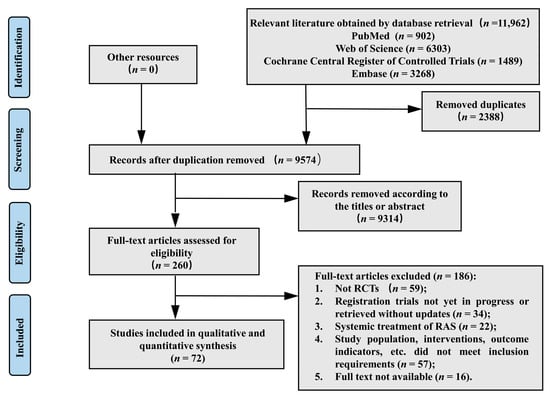 | Figure 1. Flow chart of the study selection process |
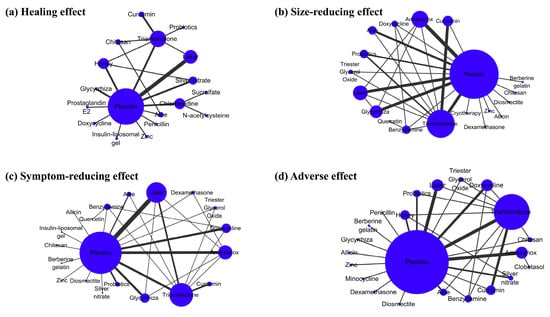
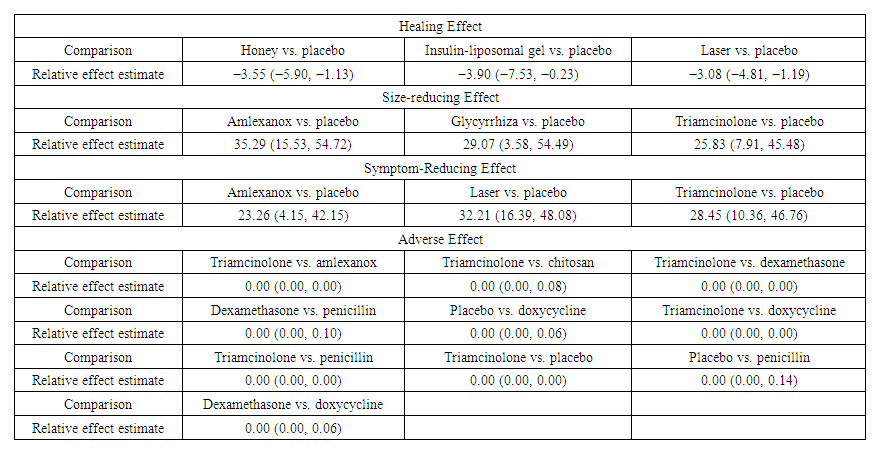 | Table 1. Significantly different estimates for healing effect |
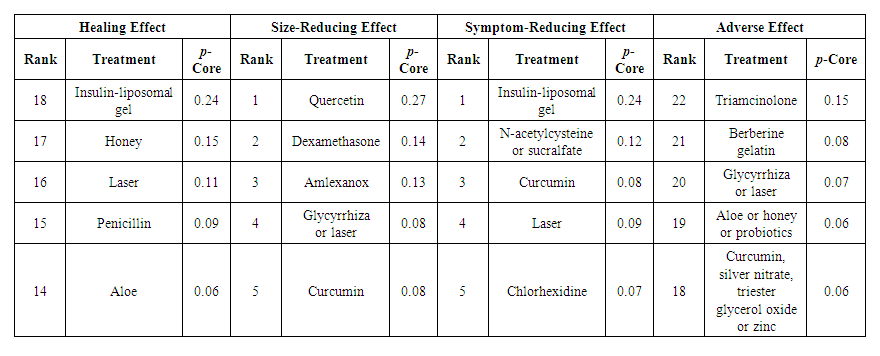 | Table 2. Outcome p-cores for the best five interventions |
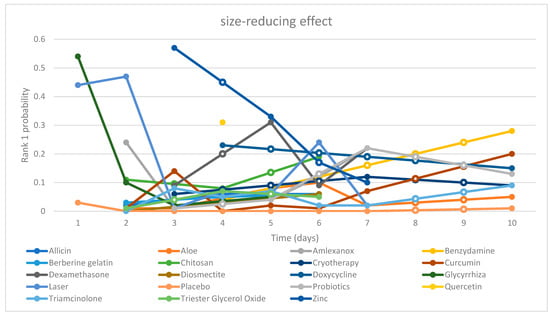 | Chart 1. “Time-Rank 1 probability” folding line chart of side-reducing effect |
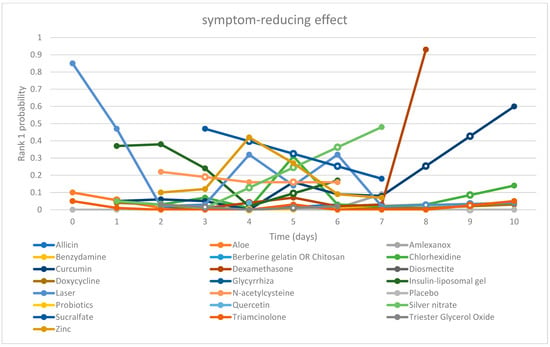 | Chart 2. “Time-Rank 1 probability” folding line chart of symptom-reducing effect |
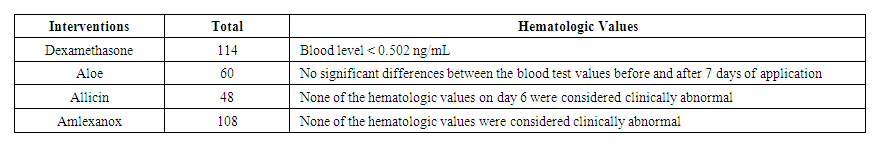 | Table 3. Hematologic values |
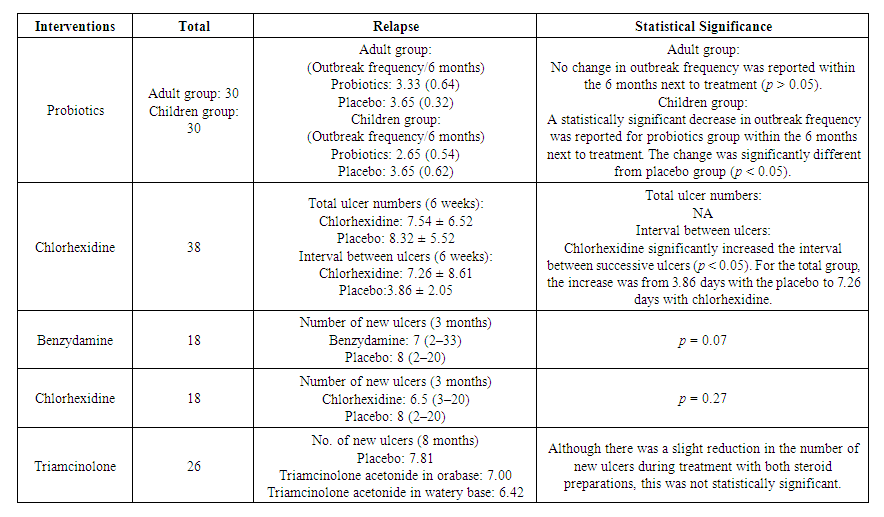 | Table 4. Recurrence and statistical significance |
4. Discussion
- Recurrent aphthous stomatitis (RAS) exhibits inherent self-healing properties [99]. However, due to its clinical features of frequent recurrence, local tissue damage, and pain [31], interventions are commonly employed. Given the unclear etiology and significant individual variability, the treatment of RAS primarily revolves around symptomatic management, with the overarching goals of promoting healing, alleviating pain, and reducing recurrence [13]. Local interventions are frequently utilized in patients with minor RAS and major RAS unaccompanied by systemic symptoms [100]. A plethora of local interventions are available, many of which have shown promising outcomes in clinical trials compared to placebo. Yet, the optimal intervention remains elusive, necessitating a thorough assessment and comparison of available topical treatments.This study represents the first systematic evaluation and network meta-analysis of topical interventions for RAS, encompassing a broad array of potential options. Both pairwise analyses and network comparisons were employed, supplemented by descriptive analyses for outcome indicators with limited sample sizes and varied evaluation metrics. A total of 29 topical interventions, including placebo, were scrutinized, ranging from allicin to zinc. Notably, clobetasol and minocycline were solely evaluated for adverse events and were not part of the primary outcome analysis. To maximize data acquisition, we conducted comprehensive searches across four major databases, complemented by gray literature sources. We established stringent inclusion and exclusion criteria, particularly excluding traumatic ulcers, radiological ulcers, and ulcers associated with systemic diseases. Seventy-two studies involving 5272 subjects were ultimately included in the analysis. Our assessment focused on four efficacy outcomes and one safety outcome. Healing effect, size-reducing effect, and symptom-reducing effect served as the primary efficacy evaluation metrics, with relapse analysis limited to descriptive examination. Safety evaluation primarily centered on adverse effects, with hematologic values serving as supplementary indicators. Additionally, rank possibility, based on p-score, served as a screening tool to aid in intervention selection.As delineated in the findings, amalgamating the four efficacy assessments along with an additional safety appraisal, we advocate our recommendation to clinicians: employ laser therapy as a short-term "shock therapy" during episodes of ulcer exacerbation, and utilize probiotics as a long-term "modifier" throughout the entire ulcer cycle.Laser therapy stands out as a pivotal milestone in clinical practice for treating oral mucosal ailments. Its efficacy extends beyond common oral mucosal conditions to encompass precancerous lesions like oral mucositis resulting from radiotherapy and chemotherapy, oral submucosal fibrosis, oral lichen planus, oral leukoplakia, and burning mouth syndrome. The therapeutic effect of laser on the oral mucosa is attributed to its stimulating biological impact. Controlled laser light within specific wavelength and power parameters engages in local metabolic processes through various physicochemical mechanisms, thereby exerting analgesic, anti-inflammatory, and pro-repair effects without causing thermal damage. Undoubtedly, its role in treating RAS is gaining recognition. Current laser treatment modalities include carbon dioxide laser, crystal laser, diode laser, and low-level laser therapy (LLLT). Our evaluation encompassed 14 randomized controlled trials (RCTs) involving laser interventions. Among these, five studies utilized diode lasers, six employed CO2 lasers, one utilized Er, Cr: YSGG lasers, one used Nd: YAG lasers, and one involved LLLT. Variations in laser modality, wavelength, and power output may impact efficacy, yet this aspect remains underexplored due to limited studies and a lack of treatment standards. Consequently, we grouped different laser modalities under the umbrella term "laser" in our study. Across efficacy evaluations, lasers emerged as superior in promoting healing, reducing ulcer size, and alleviating symptoms throughout the treatment duration. Notably, in daily assessments, laser therapy demonstrated unparalleled short-term efficacy. Furthermore, laser therapy boasts impeccable safety credentials. A recent retrospective evaluation by Valerie G. A. Suter and colleagues echoed similar expectations for laser interventions in RAS while highlighting concerns regarding laser standardization. The advantages of laser therapy over other topical interventions include: (a) short treatment duration, often yielding significant outcomes with minimal interventions; (b) superior efficacy in accelerating wound healing and pain reduction; and (c) proven safety profile, with several retrospective studies reporting no significant adverse events or hematologic abnormalities. Hence, laser therapy presents a compelling option for routine cases, steroid-intolerant individuals, and patients with severe ulcers necessitating systemic treatment. We advocate for laser therapy's use as a short-term intervention during ulcer exacerbations to expedite healing and alleviate pain. The oral cavity functions as a natural habitat for various microorganisms, with probiotics playing a crucial role. These beneficial microorganisms contribute to the construction of the oral microbial community, which is vital for maintaining oral microecological balance and counteracting pathogenic bacteria. Maintaining this delicate microbial balance is essential for oral health, as imbalance may predispose individuals to conditions such as dental caries, periodontal disease, and fungal infections. Numerous studies support the use of probiotics, either alone or in combination with other agents, to modulate the composition and structure of oral flora in diseased states, thereby intervening in conditions like dental caries, periodontal disease, and halitosis. This also extends to recurrent aphthous stomatitis (RAS). Despite the unclear etiology of RAS, evidence from microbiological and immunological studies suggests the involvement of microbial factors in its pathogenesis [10,127,128]. Consequently, probiotic therapy has been explored and implemented in RAS management [129].In our study, probiotics were administered topically for RAS management, with four randomized controlled trials (RCTs) involving Lactobacillus and one with Bacillus Clausii probiotics. While one trial utilized a mouthwash containing lactic acid, the others utilized solid tablets, with trial durations ranging from 7 to 90 days. Initially, probiotics did not demonstrate significant advantages in terms of healing, size reduction, or symptom alleviation. However, by day 7, they began to exhibit efficacy in promoting healing. Regarding recurrence, one study by Lotfy Aggour and colleagues [61] investigated the use of L. acidophilus lozenges in adult and pediatric subjects compared to placebo. They observed a significantly lower frequency of outbreaks in children using probiotics compared to controls, although this effect was not observed in adults. Notably, further studies on recurrence are warranted. Safety evaluations yielded satisfactory results. Potential mechanisms underlying the involvement of probiotics in RAS management include: (a) competition mechanism, wherein probiotics outcompete pathogenic microorganisms, leading to the establishment of harmless or beneficial biofilms [130,131,132]; (b) pro-repair effects, involving the reduction of pro-inflammatory cytokines and promotion of local tissue repair [133,134]; and (c) regulation of the microenvironment, achieved through the secretion of metabolites and active molecules that modulate the local physicochemical and immune environments, thereby promoting tissue resistance and repair [131,133,135]. These effects collectively contribute to the regulation of the microenvironment conducive to RAS, thereby reducing recurrence. However, the limited short-term healing promotion may be attributed to insufficient metabolites and active substances.Based on these considerations, we recommend the use of probiotics as a long-term intervention during both the exacerbation and remission phases of ulcers to prolong the inter-episode interval and reduce recurrence.Nevertheless, our study has several limitations. Firstly, the scarcity of RCTs on certain interventions compromises the study's credibility. Secondly, grouping different types of lasers with varying wavelengths into a single intervention category precluded exploring their differences. Additionally, more studies investigating RAS recurrence are warranted. The evidence supporting conclusions about probiotics is weakened by non-direct evidence articles and a limited number of clinical trials. Thus, future high-quality studies with larger sample sizes and standardized outcome evaluation criteria are imperative.In summary, this extensive network meta-analysis, which takes into account various considerations, indicates that the majority of local interventions did not demonstrate significant disparities in efficacy or safety. From the evidence available, we suggest employing laser therapy for short-term intervention to enhance healing and alleviate pain during the active phase of RAS, while recommending probiotics for long-term use to extend the interval between episodes and mitigate recurrence throughout both active and remission phases of RAS. We advocate for further large-scale RCTs adhering to rigorous standards to enhance the credibility of future research in this domain.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML