-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(5): 1447-1450
doi:10.5923/j.ajmms.20241405.67
Received: Apr. 23, 2024; Accepted: May 20, 2024; Published: May 30, 2024

Carbohydrate Metabolism Disorders in Patients with COVID-19
Mirzaeva U. Z.1, Nasirova Kh. Kh.1, Shariksieva M. A.2, Sadikova D. Sh.2, Shamuratova M. Sh.2
1Tashkent Pediatric Medical Institute, Uzbekistan
2Republican Specialized Scientific-and Practical Medical Centre of Endocrinology, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Components of metabolic syndrome such as carbohydrate metabolism, type 2 DM and obesity are common and significantly increase the risk of hospitalization and mortality in patients with COVID-19. Metabolic syndrome (MS) as a symptom complex leading to damage to the cardiovascular system, as well as increasing the risk of death of patients, was described in the second half of the 20th century. The main components of MS are abdominal obesity, impaired carbohydrate metabolism, arterial hypertension (AH) and lipid metabolism disorders. According to the results of the INTERHEART study, 26% of the world's population suffers from MS. In addition, MS has a steady upward trend. In 2019, a new challenge was thrown to humanity in the form of a coronavirus infection (SARS-CoV‑2), which quickly reached the level of a pandemic. Even when the first patients appeared, it was clear to doctors and scientists that the risk of severe course and death of this infection is higher in comorbid patients suffering from concomitant diseases such as obesity, diabetes mellitus, hypertension, and other lesions of the cardiovascular system. At the same time, the risk of developing severe pneumonia in patients with normal weight was significantly lower than in those with overweight or obesity. The purpose of this literature review is to study the relationship of the metabolic syndrome and its components with susceptibility to infection, as well as the features of the course of a new coronavirus infection in patients with metabolic syndrome.
Keywords: COVID-19, Carbohydrate metabolism, Hyperglycemia
Cite this paper: Mirzaeva U. Z., Nasirova Kh. Kh., Shariksieva M. A., Sadikova D. Sh., Shamuratova M. Sh., Carbohydrate Metabolism Disorders in Patients with COVID-19, American Journal of Medicine and Medical Sciences, Vol. 14 No. 5, 2024, pp. 1447-1450. doi: 10.5923/j.ajmms.20241405.67.
Article Outline
1. Relevance
- In December 2019, a series of unexplained cases of pneumonia was registered in China. Subsequent research identified a new strain of coronavirus - SARS-CoV-2, which is the causative agent of the acute infectious disease Coronavirus Disease 2019 (COVID-19) [1,4]. In a short period of time, the epidemic of the new coronavirus infection escalated into a pandemic, affecting more than 200 countries worldwide. The causative agent of the coronavirus infection COVID-19 is the SARS-CoV-2 virus, a new coronavirus that was first detected in Wuhan, China, in December 2019. The COVID-19 pandemic has turned into a public health crisis, placing a significant burden on healthcare systems. Patients with concomitant metabolic dysfunction, such as type 2 diabetes and obesity, are at a higher risk of COVID-19 complications, including multi-organ dysfunction, secondary to impaired immune response and cellular energy deprivation. About 50% of those who died from COVID-19 had metabolic and vascular disorders. It is noteworthy that there are many direct links between COVID-19 and the metabolic and endocrine systems. Thus, not only patients with metabolic dysfunction (e.g., obesity, hypertension, non-alcoholic fatty liver disease, and diabetes) are at an increased risk of developing severe COVID-19, but also SARS-CoV-2 infection can lead to the onset of new cases of diabetes or exacerbation of pre-existing metabolic disorders.It is known that the main target organs for the SARS-CoV-2 virus are the epithelial cells of the respiratory tract mucosa and the gastrointestinal tract [2]. However, the SARS-CoV-2 virus uses the ACE2 receptor to penetrate cells, which is expressed not only in the lungs but also in many other organs and tissues, including the pancreas, both in its exocrine part and in the islets of Langerhans [1]. According to the studies by Yan Y. et al., the binding of the virus to the ACE2 receptor on beta cells of the pancreas may lead to impaired function and the development of hyperglycemia [3]. On the other hand, it is assumed that uncontrolled hyperglycemia leads to glycosylation of the viral spike protein and the ACE2 receptor, thereby increasing the number of binding sites for SARS-CoV-2 with target cells, which explains the higher percentage of patients with carbohydrate metabolism disorders (CMD), including diabetes, infected with SARS-CoV-2 and experiencing a more severe course [4].Furthermore, in patients with COVID-19 and diabetes compared to those without carbohydrate metabolism disorders (CMD), higher levels of serum biomarkers associated with inflammation were observed, such as IL-6, C-reactive protein, serum ferritin, and D-dimer, indicating a more significant role of hyperglycemia in exacerbating the inflammatory response, potentially leading to the development of a "cytokine storm" [3]. Simultaneously, pro-inflammatory cytokines contribute to decreased insulin sensitivity, thereby increasing insulin resistance in peripheral tissues and aggravating hyperglycemia [5].Moreover, one of the factors contributing to CMD in COVID-19 is stress hyperglycemia, triggered by the release of counter-regulatory hormones, observed in both infectious diseases and acute pathological conditions [6]. According to MacIntyre E. et al., the prevalence of stress hyperglycemia among 2124 hospitalized pneumonia patients in Canada in 2012 was 67%, with 14% of them being diagnosed with diabetes after 5 years [7]. In a study by Ilias I. et al., among 36 patients with SARS-CoV-2 without a history of diabetes hospitalized in the intensive care unit in Athens, Greece, hyperglycemia was detected in 55.5% of cases [7]. The pathogenesis of the disease and its impact on the body are extensively discussed in both domestic and foreign literature. Observations by Chinese, American, and Italian physician-researchers indicate a more severe disease course in individuals with chronic conditions, particularly diabetes. However, emerging data suggest a potential impact of the novel coronavirus SARS-CoV-2 on carbohydrate metabolism: cases of stress hyperglycemia (SH) associated with COVID-19 have been reported [8].SH is a syndrome characterized by elevated blood glucose levels occurring in various stressful conditions in individuals without pre-existing carbohydrate metabolism disorders. Stressful conditions encompass extensive surgical interventions, acute illnesses, massive trauma, as well as certain infectious diseases. The study of SH is of great interest, firstly, due to its association with higher mortality and a greater number of complications in critically ill patients. Secondly, because the detection of overt hyperglycemia often leads to erroneous confirmation of diabetes diagnosis and subsequent initiation of therapy, which may result in hypoglycemia and other adverse effects. Additionally, stressful conditions may lead to an imbalance in carbohydrate metabolism and not only transient SH but also, subsequently, diabetes development [9].The etiological agents of coronavirus infection have been well established. All SARS viruses are characterized by their ability to enter target cells via the angiotensin-converting enzyme 2 (ACE2) receptor [10]. One of the earliest studies exploring the association between SARS coronavirus and carbohydrate metabolism was conducted in China in 2009. This prospective study enrolled 39 patients without diabetes and with no history of steroid therapy, who were hospitalized due to laboratory-confirmed coronavirus pneumonia. Among these 39 patients, 20 (51%) exhibited hyperglycemia that persisted for several days. However, glucose levels normalized by the end of hospitalization for all patients. A follow-up assessment of carbohydrate metabolism status in these patients was conducted three years later, revealing that only 2 out of 39 individuals (5%) had developed diabetes, confirming the transient nature of the hyperglycemia observed in the context of coronavirus infection, known as stress hyperglycemia (SH). Immunohistochemical examination of lung, heart, kidney, and pancreatic biopsy specimens from a 42-year-old patient who succumbed to coronavirus pneumonia revealed ACE2 expression in all examined organs, including the pancreas. Notably, the expression level of ACE2 was higher in the endocrine part than in the exocrine part [11,12]. Given the shared mechanism of SARS virus entry, it is plausible that hyperglycemia may also manifest in COVID-19. Early reports from Wuhan, China, during the ongoing pandemic indicate that 51% of patients with the new coronavirus infection developed SH. According to recent published data, among 1122 hospitalized COVID-19 patients without a history of diabetes or steroid therapy, 257 patients (22.9%) exhibited SH, confirmed by a sustained increase in capillary blood glucose levels above 9.9 mmol/L (180 mg/dL) for several days [13,14]. Another observational study involving 85 patients in the United States also demonstrated the development of SH in hospitalized patients [15]. Therefore, hyperglycemia detected in COVID-19 patients early on appears to be quite common. Presently, due to the short duration since the onset of the pandemic and the lack of prospective studies on patients with SH, the question regarding the development of diabetes induced by SARS-CoV-2 remains unanswered. Only one clinical case of diabetes onset and ketoacidosis development has been reported in a 37-year-old patient with no prior history of carbohydrate metabolism disorders or steroid use. However, the elevated HbA1c level of 14.2% suggests that SARS-CoV-2 likely triggered the manifestation of the disease rather than causing its development [16]. Recent reports from Chinese researchers have shown not only the occurrence of hyperglycemia during illness but also increased HbA1c levels (>6.0%) in many patients with no history of diabetes. These HbA1c levels were found to correlate with markers of inflammation, hypercoagulability, and blood oxygen saturation (SaO2) in COVID-19 patients [16]. Nonetheless, it should be noted that elevated HbA1c levels may be associated not only with hyperglycemia but also with iron deficiency, vitamin B12 deficiency, and certain medication use [17]. Additionally, changes in HbA1c levels may occur due to the development of methemoglobinemia, which has been identified in COVID-19 patients [18].The impact of SARS-CoV-2 on pancreatic β-cells remains inadequately investigated. Besides the direct cytotoxic effects resulting from viral replication, there may be indirect harm to pancreatic β-cells due to reduced ACE2 expression on their surface following SARS-CoV-2 penetration. ACE2's protective role on β-cell function involves increased angiotensin 1-7 activity and decreased angiotensin 2 activity, leading to β-cell apoptosis, reduced differentiation, free radical generation, and local inflammation. Consequently, decreased ACE2 expression on β-cell surfaces post-SARS-CoV-2 penetration may decrease β-cell functional activity and cause insulin insufficiency (see Fig. 1).
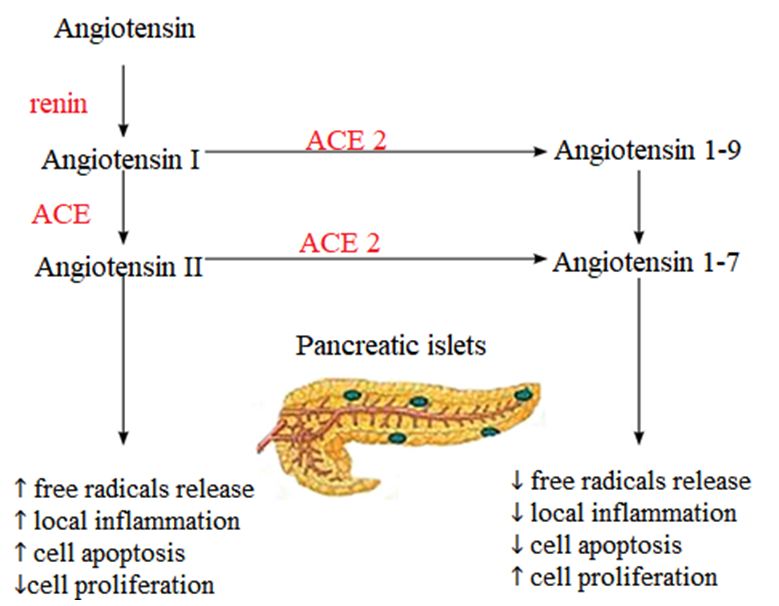 | Figure 1. Mechanism depicting the protective effect of angiotensin-converting enzyme 2 on pancreatic β-cells |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML