-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(5): 1443-1446
doi:10.5923/j.ajmms.20241405.66
Received: Apr. 24, 2024; Accepted: May 20, 2024; Published: May 30, 2024

Efficacy of “Pomalidomide” Containing Polychemotherapy Regimens in the Treatment of Relapsed Forms of Multiple Myeloma
Gulchekhra Makhamadalieva1, Khamid Karimov2, Abdurakhmon Kaumov3
13-Clinical Hematology Department, Republican Specialized Scientific-Practical Medical Center of Hematology MoH RUz, Tashkent, Uzbekistan
2Department of Molecular Medicine and Cell Technologies, Republican Specialized Scientific-Practical Medical Center of Hematology MoH RUz, Tashkent, Uzbekistan
3Administration, Republican Specialized Scientific-Practical Medical Center of Hematology MoH RUz, Tashkent, Uzbekistan
Correspondence to: Gulchekhra Makhamadalieva, 3-Clinical Hematology Department, Republican Specialized Scientific-Practical Medical Center of Hematology MoH RUz, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The presented article is devoted to the issues of clinical efficacy and safety, the specifics of the practical use of pomalidomide in patients with recurrent multiple myeloma. The article presents the results of the main clinical studies of pomalidomide-containing Pd and PVd treatment programs in treated patients with a diagnosis of multiple myeloma in RSSPMC of Hematology. The effectiveness of treatment regimens for recurrent MM based on pomalidomide is discussed.
Keywords: Multiple myeloma, Recurrent multiple myeloma, Pomalidomide, Dexamethasone
Cite this paper: Gulchekhra Makhamadalieva, Khamid Karimov, Abdurakhmon Kaumov, Efficacy of “Pomalidomide” Containing Polychemotherapy Regimens in the Treatment of Relapsed Forms of Multiple Myeloma, American Journal of Medicine and Medical Sciences, Vol. 14 No. 5, 2024, pp. 1443-1446. doi: 10.5923/j.ajmms.20241405.66.
Article Outline
1. Introduction
- Relevance. Multiple myeloma (MM) is the 2nd most frequently detected oncohematological pathology, accounting for about 10% of blood system tumors [1,6]. Despite the successes achieved in recent years in the treatment of MM and the increase in overall survival, the disease is considered incurable and is characterized by alternating remissions and relapses. The use of new drugs, including immunomodulatory drugs (lenalidomide, pomalidomide), has increased overall survival from 3 years to 7-10 years or more, but the 10-year survival rate does not exceed 17%. “Pomalidomide”, an analog of the drug “Thalidomide”, was approved by the FDA on February 8, 2013 [1,2,5,8]. According to the literature [1,4,8,10], the drug is more effective than “Thalidomide” and “Lenalidomide” and is a more potent 3rd generation IMiDs, which has an antiproliferative effect on tumor plasma cells. Immunomodulatory effect of the drug is realized by reducing the activity of regulatory T-lymphocytes and inhibiting the production of proinflammatory cytokines by monocytes: TNF-alpha and IL-6 [3,4,7,9]. According to Votyakov O.M. that the main target of the drug “Pomalidomide” is a protein - Cereblon. “Pomalidomide” binds to this target and inhibits the activity of ubiquitinligase, is also an inhibitor of COX2 transcription. In addition, due to anti-angiogenic and anti-inflammatory action “Pomalidomide” promotes reprogramming of the microenvironment. The synergistic effect of the combination of “Pomalidomide” with the drug “Dexamethasone” seems to be important for clinical practice [8,10]. Since MM is characterized by a chronic course, the search for new treatment protocols at each subsequent relapse of the disease is critical to increase overall survival.
2. Main Body
2.1. Purpose of the Study
- Evaluate the efficacy of "Pomalidomide" containing polychemotherapy regimens in the treatment of relapsing forms of multiple myeloma.
2.2. Material and Methods of Investigation
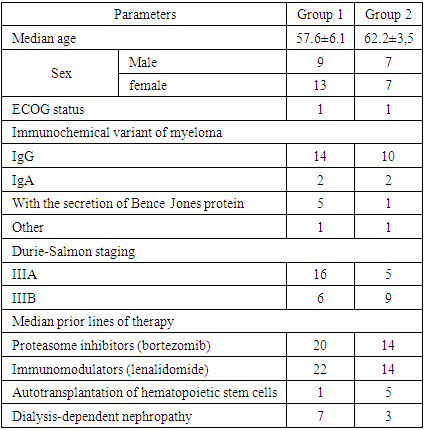
- In "Republican specialized scientific-practical medical center of Hematology of MH RUz" the analysis of the results of using pomalidomide containing treatment programs Pd ("Pomalidomide"/ "Dexamethasone"), PVd ("Pomalidomide"/ "Bortezomib"/ "Dexamethasone") in treated patients with the diagnosis of Multiple Myeloma was carried out. Thirty-six patients with reliably diagnosed multiple myeloma aged 34 to 72 years were examined, including 22 women (61%) and 14 men (39%). The largest number of patients were women aged over 60 years.We treated 36 patients with relapsed course of Multiple Myeloma who were resistant to 1,2 line of therapy, 10 of them were patients with dialysis-dependent myeloma nephropathy.The median time from diagnosis to patient inclusion in follow-up was 16 months (7 months to 32 months).The effectiveness of multiple myeloma treatment was evaluated according to international criteria [2,7] based on the assessment of laboratory parameters after each course of therapy (indices of general blood analysis, bone marrow, blood biochemical analysis, immunochemical study of monoclonal immunoglobulins) and further once every three months.The immunochemical method was used to determine the type and amount of immunoglobulin heavy (IgG, A and rare types) and light chains (κ, λ) and free chains (κ, λ) in blood serum by immunofixation of pathological monoclonal immunoglobulin and electrophoresis was determined with the help of the automated system "InterlabPretty" (Interlab, Italy) using reagents of the same company before treatment, after each course and after 3 courses of therapy (Fig. 1).
 | Figure 1. Semi-automatic device for electrophoresis of blood plasma proteins "Interlab Pretty" |
|
2.3. Results and Discussion
- Clinical examination of patients after therapy in both subgroups showed a significant decrease in the percentage and intensity of ossalgia, infectious complications and extramedullary manifestations. No new pathologic fractures were observed among the patients. At the same time, improvement of renal function in MM patients was noted.The PVd group showed a higher incidence of neutropenia (22% vs. 7%), infections (20% vs. 8%), and peripheral sensory neuropathy (9% vs. 1%) than the Pd group (Fig. 2).
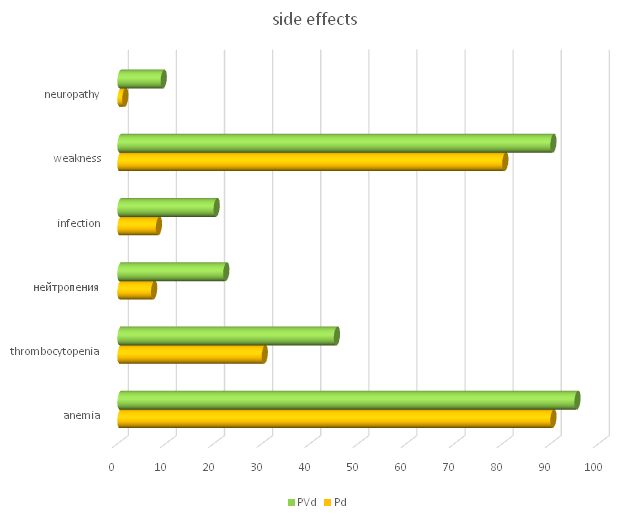 | Figure 2. Side effects of therapy in the studied groups |
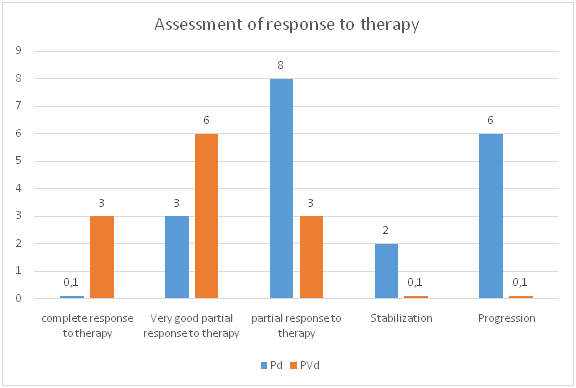 | Figure 3. Degree of response to therapy for multiple myeloma in the study groups |
3. Conclusions
- Summarizing the above data, we can conclude that the treatment performed in both groups indicates improvement of both clinical and laboratory status of MM patients. However, the dynamics of the studied clinical and laboratory parameters after treatment in the "II" subgroup was characterized by the greatest recovery in comparison with the "I" group, which was expressed clinically in the disappearance of characteristic symptoms of MM, laboratory greater increase in hemoglobin, and platelets, the greatest decrease in the level of total protein, microglobulins. All this directly convincingly proves the great effectiveness of PVd protocol application. It can be concluded that the use of pomalidomide-containing programs in patients with relapsing multiple myeloma showed high efficacy.This drug can be recommended for patients with multiple myeloma, including those with advanced renal failure. Polychemotherapy according to the PVd protocol consisting of bortezomib, pomalidomide and dexamethasone is effective compared to the Pd protocol. Further studies to determine the role of pomalidomide in the treatment of patients not only with relapsed MM, but also in patients with newly diagnosed myeloma.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML