-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(5): 1437-1442
doi:10.5923/j.ajmms.20241405.65
Received: Apr. 22, 2024; Accepted: May 21, 2024; Published: May 30, 2024

Characteristics of Vegetative Disorders in Parkinson's Disease Patients with COVID-19
Rustambek J. Matmurodov, Bekzod A. Muminov, Khanifa M. Khalimova
Department of Neurology and Medical Psychology, Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Rustambek J. Matmurodov, Department of Neurology and Medical Psychology, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background. The clinical course of Parkinson's disease in patients with Covid-19 is unique, and autonomic disorders, which are non-motor symptoms, are of particular importance. The analysis of scientific literature showed that 20-63% of cardiovascular disorders, 20-97% of gastrointestinal disorders, 60-92% of urinary disorders, 20-34% of skin-trophic disorders, and 10-50% of thermoregulatory disorders occur in PD. Methods. Characteristics of autonomic disturbances in Parkinson's disease were compared in patients with and without Covid-19. Of the total examined patients, 63 were men (50.8%) and 61 were women (49.2%). The age of the patients ranged from 22 to 78 years, and the average age was 57.2±8.3 years. The average duration of the disease was 5.7±3.5 years. Vegetative disorders are characterized by the Non-Motor Symptoms Scale (NMSS) and using the O.S. Levine scale. Results. Non-Motor Symptoms Scale (NMSS) and O.S. Levin scale results were analyzed for two groups of patients. According to the NMSS scale, hypersalivation, hyposmia, pain, memory loss, attention deficit, sadness, anxiety, and sexual dysfunction were shown to be higher in patients who underwent Covid-19 than in patients with PD. According to Levin's scale, cardiovascular disorders, urinary disorders, thermoregulatory disorders, and some components of gastrointestinal tract disorders were significantly different from those with PD in patients with Covid-19. Conclusion. The degree of vegetative disorders depends not only on the period after the transfer of Covid-19, but also on the clinical forms and stages of PD.
Keywords: Covid-19, Parkinson's disease, Vegetative disorders
Cite this paper: Rustambek J. Matmurodov, Bekzod A. Muminov, Khanifa M. Khalimova, Characteristics of Vegetative Disorders in Parkinson's Disease Patients with COVID-19, American Journal of Medicine and Medical Sciences, Vol. 14 No. 5, 2024, pp. 1437-1442. doi: 10.5923/j.ajmms.20241405.65.
1. Introduction
- The coronavirus infection (Covid-19) is a very urgent problem that has shaken the population of all the countries of the world in the form of a pandemic. There is not a single country in the world where this virus has not entered. It has caused the death of many patients, and in some patients, it has aggravated the course of certain diseases, increased disability and, in this regard, stabilized severe socio-economic problems [1,2,4,7,13,14,15,16].We know that the main target system for the infection of Covid-19 (SARS-CoV-2 virus) is the nervous system. Neurological disorders after infection with Covid-19 develop both in the acute period and after a certain period. 85% of people infected with the coronavirus experience brain fog, reduced concentration, rapid fatigue, general weakness, reduced thinking, memory loss, sleep disorders, myalgia, and anxiety-depressive disorders [5,7,9,10,19].Patients with neurodegenerative diseases of the nervous system, including PD, Alzheimer's disease, etc., have a severe infection with Covid-19. In patients with PD, new transformations of motor and non-motor disorders were shown, and this condition was related to a number of factors: the influence of general intoxication processes, respiratory failure and hypoxia on dopaminergic neurons and synaptic transmission; direct toxic effect of endotoxins; Absorption disorders and changes in pharmacokinetics of antiparkinsonian drugs against the background of diarrhea; adverse drug interactions, i.e. reduction of the effect of MAO inhibitors in the treatment of cough and other symptoms; decrease in physical activity against the background of individual isolation. It should be emphasized that PD can change its clinical characteristics even during quarantine in patients who have not had Covid-19. These include difficulty walking, impaired balance, increased tremors and decreased movement, prolonged drug-free period, cognitive impairment, anxiety-depressive disorders, sleep disorders, and autonomic disturbances [3,6,8,17].The analysis of scientific literature showed that 20-63% of cardiovascular disorders, 20-97% of gastrointestinal disorders, 60-92% of urinary disorders, 20-34 skin-trophic disorders, and 10-50% of thermoregulation disorders occur in PD [11,12,20].Constipation is one of the main symptoms in vegetative disorders and is associated with sympathetic cardiac denervation. In the late stages of the disease, disorders that lead to impairment of the quality of life and disability of patients are vegetative disorders, and in 25% of patients, vegetative disorders have a negative effect on the condition of patients compared to motor fluctuations.Cardiovascular disorders in Parkinson's disease include orthostatic hypotension, postprandial hypotension, arterial hypertension in the supine position, heart rhythm variability disorders (Pathak A., Oka Y., 2007). Gastrointestinal disorders in PD are widely reported in the scientific literature (Barone P. et al., 2009). Salivation disorders (20-89%) (Kelvin L.C., 2007), gastroesophageal symptoms (20-89%) (Djaldetti R. et al., 1996), dysphagia (22-97%) (Edward L.L. et al., 1994), constipation (29-79%) (Pfeiffer R.F., 2003) and anorectal dysfunction (30-66%) (Pfeiffer R.F., 2003). Urinary tract disorders (47-87%) (Soler J.M., 2004), obstructive urinary disorders with decreased libido (2-3%) (Araki I., Kitahara M., 2000), lack of sexual satisfaction, erection and ejaculation disorder (41-79%) (Bronner G. et al., 2004).In this regard, the above-mentioned points require a comparative analysis of the clinical course of autonomic disorders in patients with PD with and without Covid-19 and the development of criteria for a differential approach.Our research aim is to study the characteristics of autonomic disturbances in patients with Parkinson's disease who underwent Covid-19.
2. Materials and Methods
- 124 patients suffering from various clinical forms of Parkinson's disease who are being treated in stationary and outpatient conditions in the departments of the Tashkent 7th city clinical hospital, the multidisciplinary clinic of the Center for the Development of Professional Qualifications of Medical Staff, and the National Center for Rehabilitation and Prosthetics of the Disabled were registered. In order to compare some parameters during the study, the control group consisted of 30 age-matched patients without Covid-19 PD.Of the total examined patients, 63 were men (50.8%) and 61 were women (49.2%). The age of the patients ranged from 22 to 78 years, and the average age was 57.2±8.3 years. The average duration of the disease was 5.7±3.5 years.The diagnosis of Parkinson's disease was made based on the criteria of A. Hughes et al. and the British Brain Bank (Hughes A. J. et. al. 1992). Also, the diagnosis was made on the basis of the anamnesis and signs of damage to the nervous system, on the basis of differential diagnosis on the basis of clinical-laboratory and neurovisual examinations (CT, MSCT and MRI). Disease stages were assessed according to the scale of Hoehn and Yahr (Hoehn M., Yahr M.D., 1967). Clinical forms of the disease were divided into akinetic-rigid, tremor and mixed forms.Patients with PD were divided into 2 groups. Group 1 consisted of 64 patients with PD and Covid-19 infection, and group 2 consisted of 60 patients with PD and no Covid-19 infection. 34 (53.1%) of patients in group 1 were men and 30 (46.9%) were women. Group 2 patients consisted of 29 (48.3%) men and 31 (51.7%) women. Analysis of different clinical forms of patients with Parkinson's disease in both groups was carried out according to the gender of the patients. Stages of the disease were distinguished according to Hoehn and Yahr (Hoehn and Yahr M.D. 1967).Patients included in the study underwent a complete clinical and neurological examination. Clinical forms of PD diagnosis were distinguished based on the predominance of tremors and rigidity, increased muscle tone in the plastic type, and the level of occurrence of the "cog wheel" symptom. If the patients had secondary neurodegenerative diseases, these patients were not included in the study group.Vegetative disorders are characterized by the Non-Motor Symptoms Scale (NMSS) and using the O.S. Levine scale.The obtained results were statistically analyzed using a special program package Microsoft Office Excel-2007 on a Pentium-IV computer using the variational statistical method designed for medical-biological examinations. Arithmetic mean (M), mean square deviation (σ), mean standard error (m), relative quantity (incidence rate %), comparative analysis of the results obtained by the Student (t) criterion, kurtosis criterion, using variational parametric and non-parametric statistics method Probable errors (r) of the main variance equality (F-Fisher's test) were studied when testing the correctness of separation into groups. r<0.05 and r<0.001 was considered as the confidence level for statistically significant change.
3. Results and Discussion
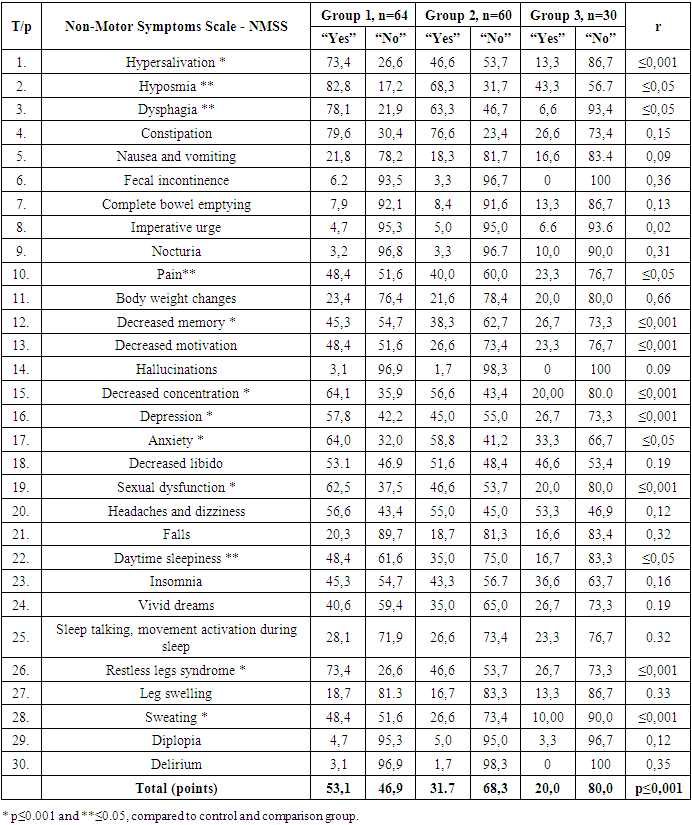
- In order to assess vegetative disturbances, the results of PD Non-Motor Symptoms Scale (NMSS) were analyzed by groups. When assessed through 30 items of this questionnaire, patients with observed disorders answer "Yes" and patients without observed "Severe". In PD patients who underwent Covid-19 in group 1, hypersalivation (73.4%), hyposmia (82.8%), dysphagia (78.1%), constipation (79.6%), nausea and vomiting (21, 8%), fecal incontinence (6.2%), complete bowel emptying (7.9%), imperative urge (7.9%), nocturia (3.2%), pain (48.4%), body weight changes (23.4%), decreased memory (45.3%), decreased motivation (48.4%), hallucinations (3.4%), decreased concentration (64.1%), depression (57.8%), anxiety (64.0%), decreased libido (53.1%), sexual dysfunction (64.1%), headaches and dizziness (56.6%), falls (20.3%), daytime sleepiness (48.4%), insomnia (45.3%), vivid dreams (40.6%), sleep talking, movement activation during sleep (28.1%), restless legs syndrome (73.4%), leg swelling (18.7%), sweating (48.4%), diplopia (4.7%), delirium (3.1%). The analysis of the obtained results shows that hypersolubilization, memory loss, motivation loss, attention loss, depression, sexual disturbances, sweating and restless legs syndrome had a higher rate in PD patients, p≤0.001, and hyposmia, constipation, pain, anxiety, daytime a reliable difference r≤0.05 was observed between sleepiness. When comparing the average indicators of general disorders on 30 points, the absence of disorders was 20.0% in the control group, and 31.7% in group 2, that is, in patients with PD but who did not have Covid-19, a reliable difference r≤0.05 was found. The difference between the overall impairment rate in PD patients who had (53.1%) and those who had not had Covid-19 was shown to be reliable, r≤0.001. The obtained results are detailed in Table 1.
|
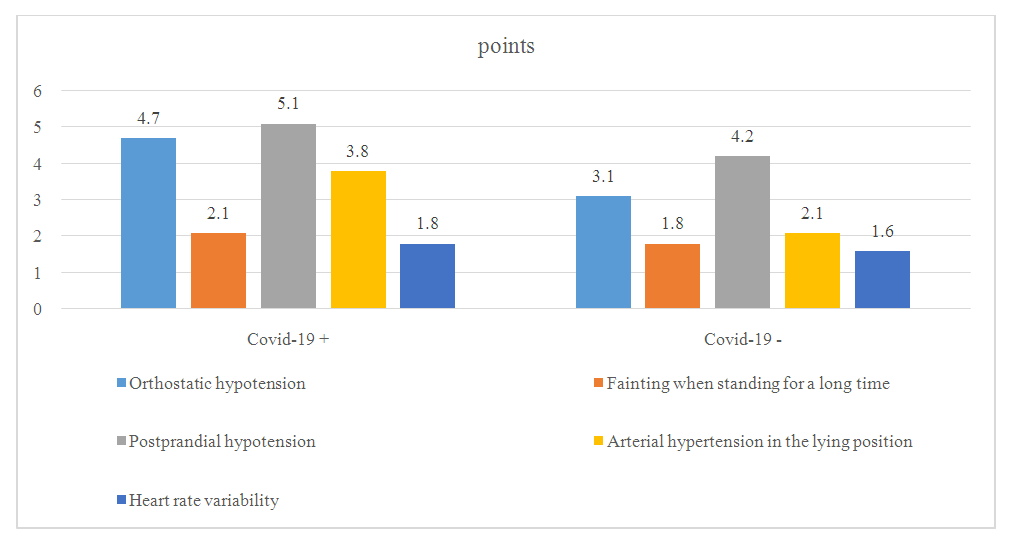 | Figure 1. Levin O.S. indicators of cardiovascular disorders according to the scale |
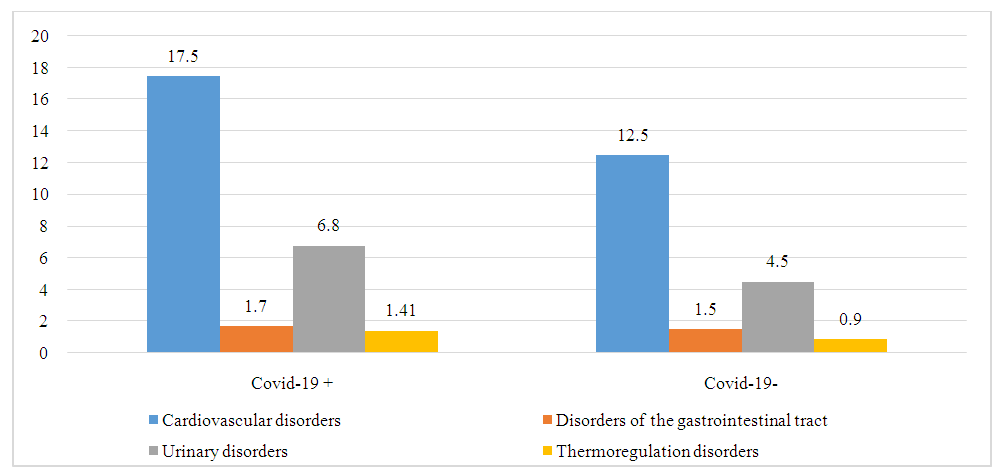 | Figure 2. Indicators of autonomic disorders in patients with and without Covid-19, score |
4. Conclusions
- Our study resulted in the following conclusions:1. Autonomic disturbances in the form of hypersalivation, hyposmia, pain, memory loss, attention deficit, sadness, anxiety, and sexual disorders were highly manifested in Parkinson's disease patients who underwent Covid-19.2. In patients with Parkinson's disease who underwent Covid-19, the observation of some components of cardiovascular disorders, urinary disorders, thermoregulatory disorders, and partial gastrointestinal tract disorders was significantly different.3. The degree of vegetative disorders depends not only on the period after the transfer of Covid-19, but also on the clinical forms and stages of Parkinson's disease.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML