-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(5): 1379-1384
doi:10.5923/j.ajmms.20241405.50
Received: Apr. 11, 2024; Accepted: May 13, 2024; Published: May 22, 2024

Comparative Evaluation of Different Treatment Approaches “Thin” Endometrium in the Preconception Period in the IVF Program
Dilnoza Saidzhalilova, Kholida Eshtemirova
Tashkent Medical Academy, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The share of the female factor in the structure of the causes of infertility reaches 42-48%, while the share of the “uterine factor” accounts for up to 60%. The frequency of detection of pathological changes in the endometrium during infertility exceeds 40%. These data support the view that the endometrium plays a key role in the processes of implantation and placentation. We examined 122 women with infertility and dysfunction (“thin”) endometrium. Depending on the method of therapy, the women were divided into 2 groups: 67 women who received estradiol valeriat and natural progesterone; and 55 women who received the heparinoid sulodexide in combination. With “thin” endometrium, there is a high frequency (56.7%) of negative results in the IVF program. Hormonal therapy helps to increase positive results in the IVF program (43.3%). Complex therapy with heparinoids ensures growth and perfusion of the endometrium and increases the effectiveness of the IVF program by 1.6 times (69.1%).
Keywords: Pathological changes, Endometrium, Hormonal therapy, Endometrium, Female factor, Uterine factor
Cite this paper: Dilnoza Saidzhalilova, Kholida Eshtemirova, Comparative Evaluation of Different Treatment Approaches “Thin” Endometrium in the Preconception Period in the IVF Program, American Journal of Medicine and Medical Sciences, Vol. 14 No. 5, 2024, pp. 1379-1384. doi: 10.5923/j.ajmms.20241405.50.
1. Introduction
- Pathological changes in the endometrium are one of the leading causes of miscarriage, not only in naturally occurring pregnancies, but also in IVF programs [1,4,6,11,17]. The share of the female factor in the structure of the causes of infertility reaches 42-48%, while the share of the “uterine factor” accounts for up to 60%. The frequency of detection of pathological changes in the endometrium during infertility exceeds 40% [2,3,13,21]. These data support the opinion about the key role of the endometrium in the implantation process [5,7,14,25].The frequency of detection of pathological changes in the endometrium during infertility exceeds 40% [8,26]. However, there is still no consensus on the endometrial indicators necessary to predict implantation in ART programs. In a number of scientific works, researchers have defined criteria for a “thin” endometrium and most opinions agree that for pregnancy to occur, the thickness of the endometrium should be at least 7 mm [4,8,12,15]. It has been established that if the thickness of the endometrium is less than 7 mm, then the probability of embryo implantation is about 15% [10,16,20], but even if pregnancy occurs, the risk of miscarriage remains high and is about 38-45% [3,6,22]. “Thin” endometrium is one of the most common reasons for implantation failure in IVF cycles. According to most researchers, the endometrium is considered “thin” if its thickness is less than 8 mm on the 19-24th day of the menstrual cycle [21,23]. Altered morphological characteristics of the endometrium negatively affect the prognosis of implantation [14,24]. With a “thin” endometrium, the likelihood of IVF success is extremely low, and the procedure is usually not performed [27]. The polyetiology of endometrial dysfunction is noted [18,19]. The condition of the endometrium is determined by factors such as age, lifestyle, the presence of gynecological diseases and endocrine disorders, among which the leading place is occupied by the hormonal factor and circulatory failure in the endometrium. If a decrease in endometrial thickness is caused by endocrine reasons, it is necessary to prescribe hormonal drugs to stimulate endometrial growth. In cryoprotocols, when the endometrium is prepared for the transfer of thawed embryos, estrogen preparations (Proginova, Estrofem, Divigel, Estrogel) are usually used, followed by the administration of progesterone (Utrozhestan, Fetalston, Crinon) [24].But sometimes there are situations when, even with hormonal therapy, the endometrium does not reach the required thickness. Inflammatory changes in gynecological diseases and iatrogenic interventions are accompanied by the development of regenerative-plastic and functional disorders: decreased perfusion and impaired receptivity. These pathological processes lead to the formation of a “thin” endometrium, which prevents normal implantation and explains the high frequency of reproductive losses in this group of patients [9].It must be remembered that the endometrium contains a huge number of blood vessels, actively supplying and nourishing important endometrial structures and cells necessary for implantation and physiological functioning of the trophoblast.A number of patients with miscarriage or infertility, even with normal hormone levels, experience insufficient growth and functioning of the endometrium [9,10]. Thus, the work of some researchers has shown that an increase in peripheral vascular resistance in the uterine vasculature serves as a trigger for a decrease in the rate of proliferation and production of vascular endothelial growth factor in the endometrium and explains the decrease in the growth rate of the glandular layer and vasculogenesis in the presence of a “thin” endometrium [9,10]. The need for sufficient thickness of the endometrium, but also for its full functioning in women with infertility to increase the pregnancy rate in IVF programs is beyond doubt, which determines the continuation of research to study the possibility of influencing these aspects of endometrial dysfunction. One of the methods of treating “thin” endometrium with an endocrine cause is growth stimulation with the help of hormonal drugs. Estrogens are necessary for endometrial proliferation and the effects of progesterone (P). At the moment, there are many methods for stimulating endometrial growth, which include hysteroscopy adhesiolysis, administration of estrogens, vasoactive agents, intrauterine administration of granulocyte colony-stimulating factor, platelet-rich autologous plasma, stimulation of regeneration by physical methods and with the help of placental preparations, etc. [5,10,20].Of all the listed methods, only the administration of estrogens and hysteroscopy adhesiolysis are included in the clinical recommendations for the preparation of the endometrium in IVF programs in the world, and the remaining methods continue to be the subject of scientific research. In preparation protocols for IVF, stimulation of endometrial growth is achieved using estrogen-containing drugs followed by the administration of progesterone. It is not uncommon that even with hormonal therapy, the thickness of the endometrium does not reach the required thickness for normal functioning. Apparently, in addition to hormonal causes of endometrial dysfunction, there is also a cause of insufficient angiogenesis and blood circulation in this functional layer.In this regard, the treatment of endometrial dysfunction in the preconception period remains controversial.Purpose of the study: in a comparative aspect, to evaluate different approaches to the treatment of endometrial dysfunction in the preconception period in the IVF program.
2. Material and Methods of Research
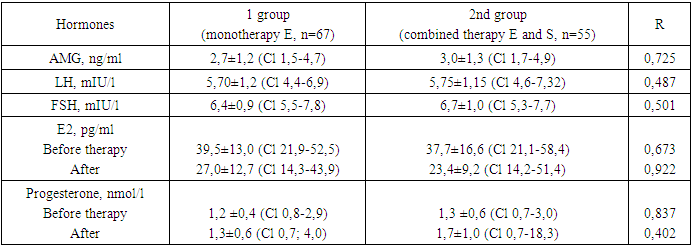
- A comparative prospective study was carried out on 122 women with infertility in an IVF program with a diagnosis of dysfunction of the (“thin”) endometrium established during the study (n=122). Depending on the method of therapy, the women were divided into groups: Group 1 consisted of 67 women who were prescribed a drug containing estradiol valeriat at a dose of 2 mg for 3 months (Proginova, France) and a natural progesterone drug (Fetalston) as therapy. , India) at a dose of 100 mg 2 times a day from 16 to 26 days of the cycle; The 2nd group consisted of 55 women who, in combination with hormonal therapy, received the heparinoid sulodexide (Wessel-due F, Italy) at a dose of 1000 units/day (2 tablets 2 times a day) for 3 months.Inclusion criteria: endometrial thickness less than 7-8 mm in the 2nd phase of the cycle, woman’s age less than 40 years, absence of other uterine pathologies (uterine endometriosis of degree II or more, uterine fibroids larger than 20 mm), severe somatic diseases, absence of contraindications to prescription of estrogens, low risk of thrombotic complications, in the future transfer of no more than 1 embryo of good quality.We assessed the state of the endometrium after the use of various therapies (endometrial thickness before ET, increase in endometrial thickness (%), the presence of perfusion), the presence of side effects from taking drugs and the outcomes of IVF programs (pregnancy rate, “take baby home” rate and the rate of reproductive losses).When included in the study at the 2nd-3rd DMC, all women underwent transvaginal ultrasound examination (TVUS) according to the standard method [15,17,18]. To determine the functional activity of the endometrium, its thickness, structure and volume, the nature of the distribution of the color Doppler mapping (CDM) signal in the endometrium and sub endometrial zone were assessed. Monitoring of endometrial growth during the menstrual cycle was carried out at the 8th, 12-14th, 19-24th DMC. On the 3rd day of the menstrual cycle (DMC), the hormonal profile of the patients was assessed. Serum concentrations of anti-Müllerian hormone (AMH), follicle-stimulating hormone (FSH), luteinizing hormone (LH) and estradiol (E2) were determined. At the stage of preparing the endometrium for embryo transfer, patients of group 1 received estradiol vale rate 2 mg (Proginova, France) in tablet form, orally, according to the schedule. Estrogen preparations were used for 3-6 months until a clinical effect was achieved in relation to the thickness and structure of the endometrium, after which an embryo transfer was performed in the subsequent menstrual cycle. In all patients, 14 days after PE, the level of β-hCG in the peripheral blood was determined, and if the value was more than 50 IU/l, the test for pregnancy was considered positive. Mathematical processing of the results was carried out using descriptive statistics methods using Excel 2010. Descriptive statistics of quantitative variables are presented as medians (Me) and quartiles (Q1; Q3). The confidence interval (CI) is indicated as M (SD), where M is the arithmetic mean and SD is the standard deviation. To determine the statistical significance of differences in quantitative variables in independent samples, nonparametric tests (Mann-Whitney test) were used. Comparative analysis of categorical variables was performed using Student's t test. Differences were considered statistically significant at r.
3. Research Results
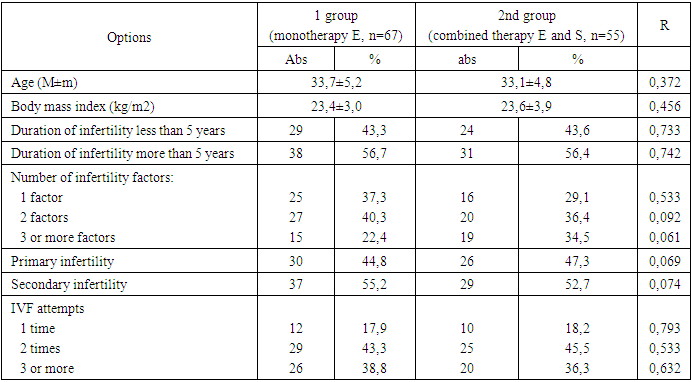
- A study of the age characteristics of women with infertility who applied for IVF showed that the average age was 33.7±5.2 and 33.1±4.8, respectively (Table 1). This is apparently due to the duration of infertility treatment with other methods. Body mass index indicators in women in the study groups were within unreliably significant limits and amounted to 23.4±3.0 and 23.6±3.9. Determining the duration of infertility in groups revealed that more than half of the women (56% and 55.4%, respectively) who turned to IVF suffered from infertility for more than 5 years.
|
|
|
4. Conclusions
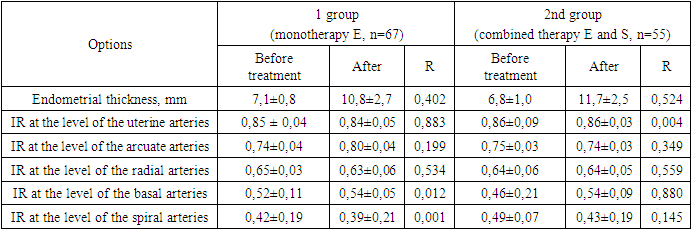
- 1. When a “thin” endometrium is formed, the implantation process is disrupted, which explains the high frequency of negative results in assisted reproductive technology programs (56.7%). Hormonal therapy increases the thickness of the endometrium, which contributes to an increase in positive results in the IVF program (43.3%).2. For “thin” endometrium, it is advisable to prescribe estrogen preparations in combination with heparinoids (sulodexide), which ensures adequate growth and perfusion of the endometrium, which increases the effectiveness of the IVF program by 1.6 times (69.1%).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML