Sidikov Akmal Abdikaxarovich1, Sadikova Asiya Maratovna2
1DSc., Professor, Fergana Medical Institute of Public Health, Uzbekistan
2Junior Researcher, Republican Specialized Scientific and Practical Medical Center of Dermatovenereology and Cosmetology, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This article presents the results of a pathomorphological study of tissues from patients suffering from Lichen planus (LP), a chronic dermatological disease affecting the skin and mucous membranes. The study aims to study detect the morphological changes and immune response in affected tissues, which is key to understanding the pathogenesis of the disease. Materials Patients and methods: The analysis was carried out on the basis of biopsy samples of skin and mucous membranes taken from 50 patients with a clinically confirmed diagnosis of LP. Specimens were processed using standard histological methods and stained using Hematoxylin and Eosin, and underwent immunohistochemical processing to identify markers of the immune response. Results: The study revealed specific pathological changes including hyperkeratosis, acanthosis and lymphocytic infiltration. Immunohistochemical analysis showed increased expression of CD4 and CD8 markers, indicating the active participation of cell-mediated immunity in the pathogenesis of LP.
Keywords:
Lichen planus, Pathomorphology, Immunohistochemistry, Histology, Immune response
Cite this paper: Sidikov Akmal Abdikaxarovich, Sadikova Asiya Maratovna, Pathological Studies of Patients with Lichen Planus, American Journal of Medicine and Medical Sciences, Vol. 14 No. 5, 2024, pp. 1332-1336. doi: 10.5923/j.ajmms.20241405.39.
1. Introduction
Lichen planus (LP) is a chronic disease of the skin and mucous membranes, characterized by a multifaceted clinical picture and a significant impact on the quality of life of patients. Despite the prevalence of this dermatological disease, its etiology is still not fully understood, and the mechanisms of development are complex and diverse. This makes lichen planus an important area for clinical and pathological research.Pathomorphological examination of tissues affected by LP plays a key role in understanding the pathogenetic mechanisms of the disease, which can contribute to the development of new methods of diagnosis, treatment and prevention. The study of morphological changes, inflammatory processes and cellular elements in lesions allows for a deeper study of the interaction of the immune system and pathologically altered tissues.Data are currently available on various aspects of LP, but many of them relate mainly to clinical manifestations and pharmacological treatment. Pathomorphological aspects are less studied, which creates a gap in a systematic and comprehensive approach to the study of the disease. Additional research in this area is necessary to confirm previously obtained data and clarify the mechanisms of development of pathological processes.Thus, the topic of pathomorphological studies of patients with lichen planus remains relevant and in demand in modern medicine, since continued scientific work in this area will help improve diagnostic capabilities and develop new approaches to the treatment of this disease.
2. Materials Patients and Methods
1. Sampling:The study is carried out on the basis of the dermatology department of the Fergana Medical Institute of Public Health. The sample includes patients diagnosed with lichen planus between January and December 2023. Inclusion criteria: presence of a clinically confirmed diagnosis of LP, consent to a biopsy of the affected skin and mucous membranes. Exclusion criteria: presence of other active skin diseases, use of immunosuppressive therapy.2. Data collection methods:Data is collected from patient medical records, including medical history, clinical data, and laboratory results. A biopsy of the affected areas of the skin and mucous membranes is performed under local anesthesia, followed by histological examination.3. Histological analysis:The biopsied material is fixed in a 10% formaldehyde solution, then undergoes a process of dehydration, transparency and embedding in paraffin. Paraffin blocks are cut on a microtome into sections 4-5 microns thick. Sections are stained with hematoxylin and eosin, as well as additional specific immunohistochemical and cytochemical methods for a detailed study of cellular infiltration and tissue structure.4. Immunohistochemistry:Antibodies to key markers of inflammation and immune response are used (eg, CD4, CD8, CD20, and CD68). This allows you to assess the nature and intensity of the immune reaction in the affected tissues.5. Statistical data processing:Data are analyzed using statistical software. Basic statistical methods include descriptive statistics, Student's t test for comparing two means, and ANOVA for multigroup analysis when appropriate. The significance level is set at p < 0.05.6. Ethical considerations:The study was conducted in accordance with ethical standards and after obtaining written informed consent from all participants. The ethics committee of the medical institution approved the study protocol.
3. Results and Discussion
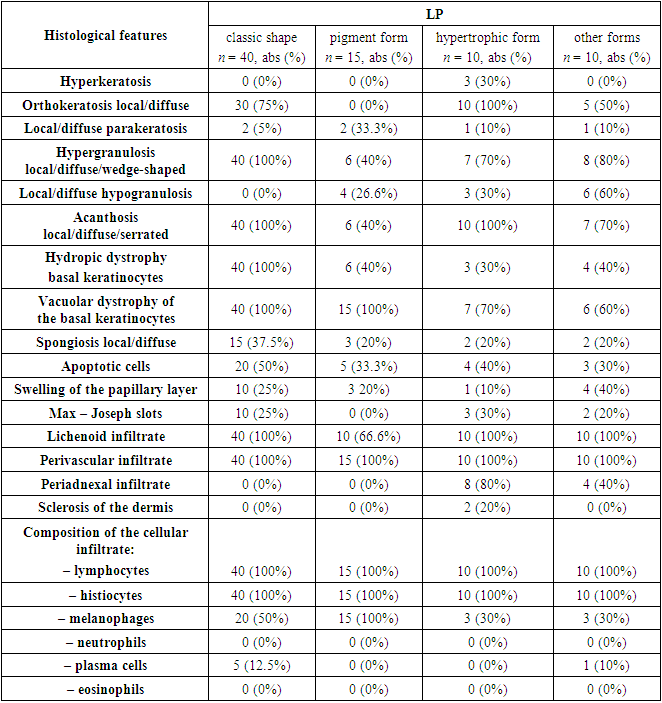
Histological examination was performed in all 80 patients in groups. All pathomorphological signs and their results are presented in Table 1 and Figure 1 For confirmation of the diagnosis, each specific case was assessed and reviewed by several dermatopathologists from the university clinic of the St. Petersburg State Pediatric Medical University of the Ministry of Health of the Russian Federation. Each histological report was correlated and compared with the clinical data of the patients. Data such as the duration of the disease, treatment received by patients, and refractoriness to therapy were taken into account. All unverified diagnoses were excluded from the study and replaced with other histological retrospective specimens. The final clinicopathological diagnosis was verified in 40 out of 80 (50%) patients with the classic form of LP. The diagnosis of “pigmented form of lichen planus” was made in 15 out of 80 (19%) people. The hypertrophic form of LP was confirmed in 10 out of 80 (12.5%) patients. Lichen planus of the scalp was detected in 3 out of 80 (4%), while LP of the oral cavity was detected in 2 out of 80 (2.5%), and forms such as atrophic, actinic, bullous, invert and nail plates, in only 1 out of 80 (1.25%) patients. Due to the rarity of the above-described forms of LP in the studied populations, the last 10 cases were included in the group “LP, other forms.” Thus, all examined patients were divided into 4 large groups.Table 1. Results of the main histological features of patients with various forms of lichen planus
 |
| |
|
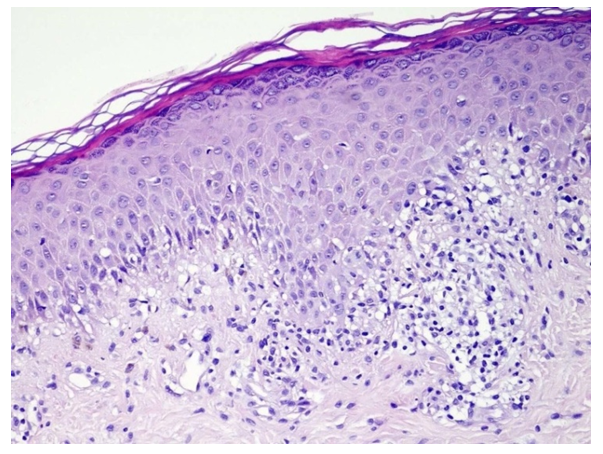 | Figure 1. Diagnosis - lichen planus, classic form. The epidermis shows orthokeratosis, hypergranulosis, vacuolar degeneration of the basal layer cells and a diffuse lichenoid infiltrate of lymphocytes and histiocytes in the papillary layer of the dermis. Hematoxylin and eosin staining. ×200 |
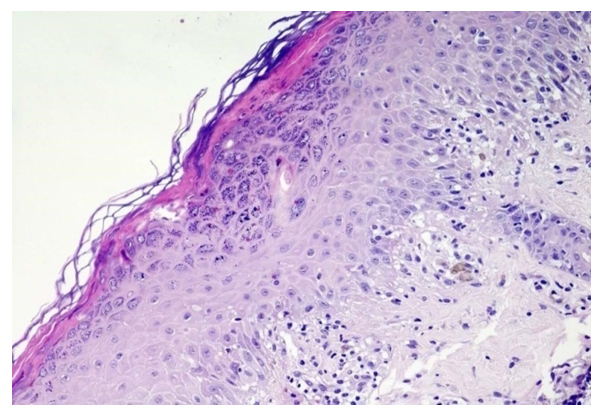 | Figure 2. Diagnosis - lichen planus, classic form. In the epidermis, orthokeratosis, wedge-shaped hypergranulosis, single apoptotic cells, vacuolar degeneration of the cells of the basal layer and lymphocytic-histiocytic infiltrate in the papillary layer of the dermis are noted. Hematoxylin and eosin staining. ×200 |
The main histological features in the epidermis of the first group were orthokeratosis throughout the entire section (n = 30/40; 75%), wedge-shaped hypergranulosis (n = 40/40; 100%), diffuse and serrated acanthosis (n = 40/40; 100%), vacuolar and hydropic degeneration of the cells of the basal layer of the epidermis (n = 40/40; 100%), intercellular edema of varying severity (spongiosis) (n = 15/40; 37.5%), the presence of apoptotic cells at the level of the spinous layer of the epidermis (n = 20/40; 55%) and in some cases - parakeratosis (n = 2/40; 5%). In the dermis, minor swelling and Max – Joseph clefts were observed in the papillary layer (n = 10/40; 25%), lichenoid (n = 40/40; 100%) and perivascular (n = 40/40; 100%) lymphocyte infiltrate (n = 40/40; 100%), histiocytes (n = 40/40; 100%) and melanophages (n = 20/40; 50%) (see Figures 3).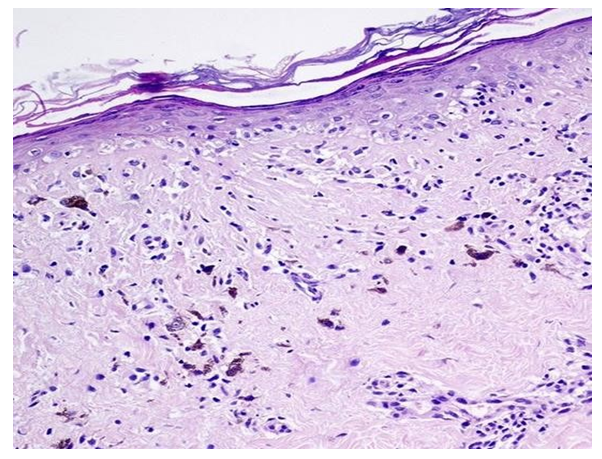 | Figure 3. Diagnosis - lichen planus, pigmented form. In the epidermis, orthokeratosis, atrophy, and vacuolar degeneration of the cells of the basal layer are observed. In the dermis, a lymphocytic-histiocytic infiltrate is observed around the vessels of the papillary layer of the dermis with multiple macrophages along the periphery. Hematoxylin and eosin staining. ×200 |
In the second group of patients with the hypertrophic form of LP, signs such as uneven and wedge-shaped hypergranulosis (n = 6/15; 40%), vacuolar (n = 15/15; 100%) and hydropic dystrophy (n = 6/15; 40%) were noted.) cells of the basal layer of the epidermis, in some cases local hypogranulosis (n = 4/15; 26.6%), uneven acanthosis (n = 6/15; 40%), in a small amount focal spongiosis (n = 3/15; 20 %), as well as apoptotic cells (n = 5/15; 33.3%). Edema was detected in the papillary dermis in 3 of 15 (20%) patients. Among the inflammatory infiltrate, perivascular (n = 15/15; 100%) prevailed over lichenoid infiltrate (n = 10/15; 66.6%), consisting of lymphocytes (n = 15/15; 100%), histiocytes (n = 15 /15; 100%) and melanophages (n = 15/15; 100%) (see Table 1, Figure 4).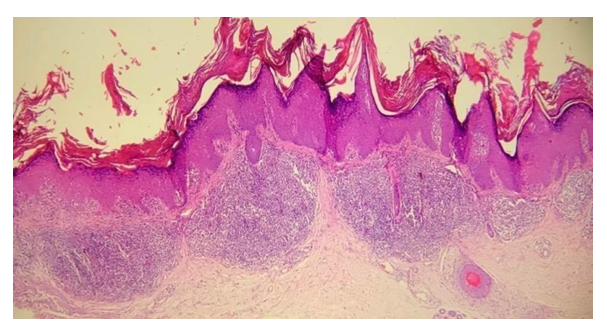 | Figure 4. Diagnosis - lichen planus, hypertrophic form. In the epidermis there is a pronounced orthohyerkeratosis, irregular acanthosis, wedge-shaped, diffuse hypergranulosis and vacuolar degeneration of the cells of the basal layer of the epidermis. In the mamillary layer of the dermis, a dense, stripe-like lichenoid mixed cellular infiltrate of lymphocytes and histiocytes will be noted. Hematoxylin and eosin staining. ×100 |
In the third group of patients, the histological signs in the epidermis included hyperkeratosis (n = 3/10; 30%), diffuse orthokeratosis (n = 10/10; 100%), local parakeratosis (n = 1/10; 10%), wedge-shaped and local hypergranulosis (n = 7/10; 70%), local hypogranulosis (n = 3/10; 30%), diffuse acanthosis (n = 10/10; 100%), hydropic (n = 3/10; 30%) and vacuolar (n = 7/10; 70%) degeneration of cells in the basal layer of the epidermis (see Figure 2), local spongiosis (n = 2/10; 20%) and a small number of apoptotic cells (n = 4/10; 40%). Due to the duration of the lesions, edema of the papillary dermis in this group was detected in only 1 of 10 (10%) patients, while Max - Joseph clefts were detected in 3 of 10 (30%) patients, respectively. In the reticular layer of the dermis, a dense lichenoid, perivascular, periadnexal infiltrate, consisting of lymphocytes, histiocytes, melanophages and single melanophages, attracted attention. All of the above histological signs were observed in 10 out of 10 (100%) patients. Only periadnexal infiltrate was observed in 8 out of 10 (80%) patients with the hypertrophic form of LP. Another pathomorphological sign was sclerosis of the dermis at the level of the reticular layer of the dermis (n = 2/10; 20%).In the fourth group, we tried to combine the remaining 10 cases together and apply them to all remaining forms of LLP. It should be noted that the bullous form occurred during biopsy from the oral cavity, and therefore the epidermis was represented by stratified squamous non-keratinizing epithelium. While the LLP of the scalp was represented by multiple hair follicles, and the mixed cellular infiltrate was predominantly located around them (see Figure 4). In all forms of the disease, the following pathomorphological signs were identified: local/diffuse orthokeratosis (n = 5/10; 50%), local/wedge-shaped hypergranulosis (n = 8/10; 80%), local hypogranulosis (n = 6/10; 60% ), varying severity and form of acanthosis (n = 7/10; 70%), vacuolar (n = 6/10; 60%) and hydropic (n = 4/10; 40%) degeneration of cells of the basal layer of the epidermis, spongiosis (n = 2/10; 20%) and apoptosis (n = 3/10; 30%) in acute courses of the disease (see Figures 5). In the dermis, swelling of the papillary layer of the dermis (n = 4/10; 40%), Max – Joseph fissures (n = 2/10; 20%), lichenoid and perivascular infiltrate (n = 10/10; 100%), in a number of cases cases, accumulations of inflammatory cells around the hair follicles were noted (n = 4/10; 40%). In all cases, the main component of the infiltrate were lymphocytic and histiocytic cells (n = 10/10; 100%).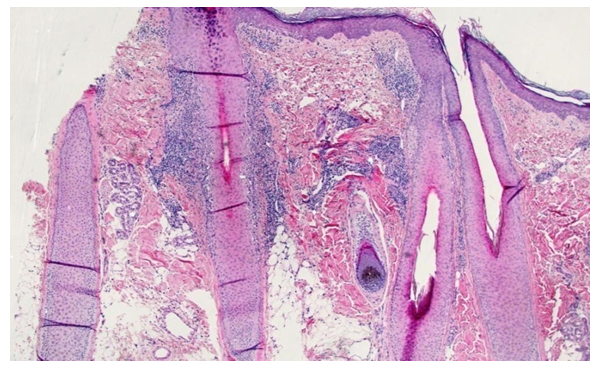 | Figure 5. Diagnosis - lichen planus of the scalp. No changes are observed in the epidermis. The granular layer of the hair follicle is hyperplastic (hypergranulosis). In the body of the hair follicle there is a dense lichenoid infiltrate of lymphocytes. Around the new follicles there are sclerotic collagen fibers. Hematoxylin and eosin staining. ×100 |
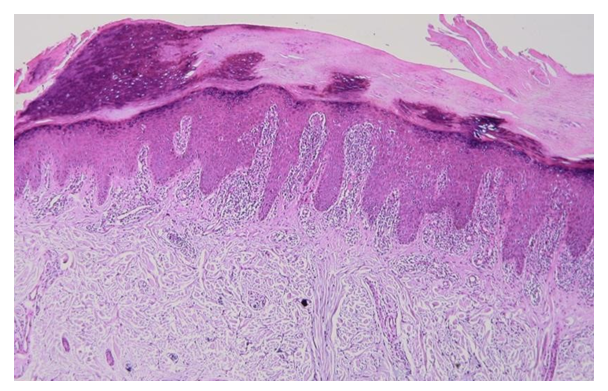 | Figure 6. Diagnosis - lichen planus of the palms and soles |
In the epidermis there is a pronounced orthohyperkeratosis, diffuse hypergranulosis, and irregular acanthosis. In the papillary layer of the dermis, a strip-like lichenoid mixed cellular infiltrate is noted. Hematoxylin and eosin staining. ×100.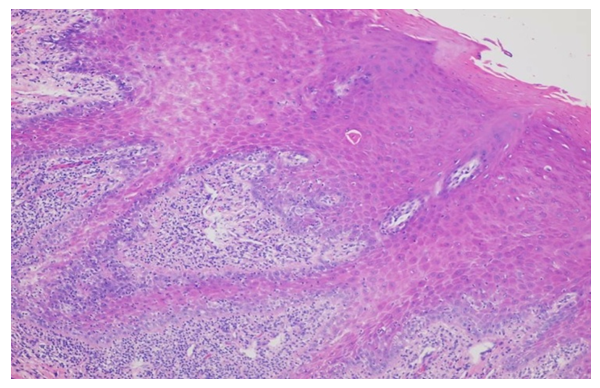 | Figure 7. Diagnosis - lichen planus of the oral cavity. Apoptotic cells and vacuolar degeneration of cells in the basal layer of the epidermis are observed in the epidermis. In the papillary layer of the dermis there is a lichenoid infiltrate of lymphocytes and histiocytes. The vessels are sharply dilated. Hematoxylin and eosin staining. ×400 |
4. Conclusions
Clinical manifestations of lichen planus are varied and are represented by flat papules, hyperpigmented spots, nodes, erosions, atrophy and alopecia with predominant localization in the flexor surfaces of the limbs, large folds, trunk and back. The facial skin remains intact.The histopathological pattern of inflammation of the classical form of LP is “ interface dermatitis, lichenoid type” (dense orthokeratosis, uneven wedge-shaped hypergranulosis, uneven acanthosis, vacuolar or hydropic degeneration of the cells of the basal layer of the epidermis, the presence of single apoptotic cells and a dense lichenoid lymphocytic-histiocytic infiltrate at the level of the epidermis small articulation), while in the pigmentary and hypertrophic forms the inflammation pattern “interface dermatitis, vacuolar type” predominates (ortho / hyperkeratosis, atrophy or acanthosis, hydropic degeneration of the cells of the basal layer of the epidermis and a less pronounced lichenoid, perivascular, interstitial infiltrate of lymphocytes, histiocytes and melanophages).Immunohistochemistry is a highly sensitive, justified, reliable method for identifying the etiological factor of the infectious process in skin biopsies of various forms of LP and is applicable for differential diagnosis in dermatopathology.
References
| [1] | Barbinov, D.V. Criteria for histological diagnosis of lichen planus / D.V. Barbinov, R.A. Ravodin // St. Petersburg dermatological readings: materials of the IV Ross. scientific-practical conf. - St. Petersburg, 2010. - P. 18. |
| [2] | Butareva, M.M. Lichen planus associated with viral hepatitis C: features of therapy / M.M. Butareva, M.B. Zhilova // Vestn. dermatology and venereology. - 2010. - No. 1. - P. 105–108. |
| [3] | Butov, Yu.S. Lichen // Clinical dermatovenerology: a guide for doctors: in 2 volumes / Yu.S. Butov, V.Yu. Vasenova, T.V. Anisimova; edited by Yu. K. Skripkina, Yu. S. Butova. - M.: GEOTAR-Media, 2009. - T. II. — pp. 184–211. |
| [4] | Gorlanov, I.A. Pediatric dermatovenerology / I.A. Gorlanov, D.V. Zaslavsky, I.R. Milyavskaya [and others]. - M.: Publishing center "Academy", 2012. - 352 p. |
| [5] | Dovzhansky, S.I. Clinic, immunopathogenesis and therapy of lichen planus / S.I. Dovzhansky, N.A. Slesarenko // Russian Med. magazine - 1998. - No. 6. - P. 348–350. |
| [6] | Epishova, A.A. Clinic, diagnosis, treatment of patients with lichen planus of the oral mucosa: abstract. dis....cand. honey. Sciences: 14.00.21 / Epishova Anna Andreevna. - Perm, 1993. - 20 p. |
| [7] | Letaeva, O.V. Clinical and pathogenetic characteristics of patients with lichen planus and rationale for therapy: abstract. dis....cand. honey. Sciences: 14.01.10 / Letaeva Olga Vladimirovna. - Ekaterinburg, 2012. - 27 p. |
| [8] | Sydikov, A.A. Clinical, morphological, immunohistochemical and molecular genetic characteristics of erythroderma: abstract. dis....Dr. med. Sciences: 14.03.02, 14.01.10 / Sydikov Akmal Abdikaharovich. - St. Petersburg, 2019. - 40 p. |
| [9] | Abbas, AK Cellular and Molecular immunology / AK Abbas, H. Lichtman. — 5th ed. - WB Saunders company publication, 2000. - P. 110–115. |
| [10] | Badri, T. Isolated genital annular lichen planus / T. Badri, N. Kenani, R. Benmously [et al.] // Acta Dermatovenerol. Alp. Pannonica Adriat. — 2011. - Vol. 20, No. 1. - P. 31–33. |
| [11] | Balasubramaniam, P. Lichen planus in children: Review of 26 cases / P. Balasubramaniam, M. Ogboli, C. Moss // Clin. Exp. Dermatol. — 2008. - No. 33. - P. 457–459. |
| [12] | Bez, C. Lack of association between hepatotropic transfusion transmitted virus infection and oral lichen planus in British and Italian populations / C. Bez, R. Hallett, M. Carrozzo [et al.] // Br. J. Dermatol. - 2001. - Vol. 145, No. 6. - P. 990–993. |
| [13] | Bilgili, SG The prevalence of skin diseases among the geriatric patients in Western turkey / SG Bilgili, AS Karadag, HU Ozkol [et al.] // J. Pak. Med. Assoc. - 2012. - No. 62. - P. 535–539. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML





