-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(5): 1151-1156
doi:10.5923/j.ajmms.20241405.02
Received: Apr. 19, 2024; Accepted: May 5, 2024; Published: May 7, 2024

Effects of Long-Term Consumption of Energy Drinks on the Heart
Sharipova Madina Akram Qizi
Bukhara State Medical Institute, Bukhara, Uzbekistan
Correspondence to: Sharipova Madina Akram Qizi, Bukhara State Medical Institute, Bukhara, Uzbekistan.
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Energy drinks (EN) are intended for young and active people and are marketed as enhancers of energy, concentration, physical and cognitive performance. Their long-term consumption causes serious health problems related to cardiovascular diseases. Here we investigate the effects of long-term consumption of Tornado energy drink on certain biochemical parameters and myocardial ultrastructure. Male rats were divided into four groups and given different treatments via oral administration. Control group (C) received tap water, Tornado group (T) received 1.5 ml/100 g body weight Tornado. In the last 6 days of the experiment, the animals were tested for physical performance by performing a forced swimming test with weights. Immediately after swimming exhaustion, animals were sacrificed under anesthesia and cardiac muscle samples were collected for ultrastructural and biochemical analysis. Our results showed a significant increase in cardiac glucose and glycogen concentrations in the T group. Total cholesterol concentrations decreased significantly in the group T. Total protein concentrations and ALT and AST activities increased in all groups. Biochemical changes were accompanied by ultrastructural changes. Based on these results, we recommend that athletes and active individuals avoid long-term consumption of energy drinks.
Keywords: Enhancers of energy, Cognitive performance, Anesthesia, Cardiac muscle samples, Glycogen concentrations
Cite this paper: Sharipova Madina Akram Qizi, Effects of Long-Term Consumption of Energy Drinks on the Heart, American Journal of Medicine and Medical Sciences, Vol. 14 No. 5, 2024, pp. 1151-1156. doi: 10.5923/j.ajmms.20241405.02.
Article Outline
1. Introduction
- Energy drinks (EN) are intended for young and physically active people. They contain large amounts of active ingredients such as caffeine, taurine and niacin. ENs are considered agents or enhancers of energy, concentration, physical and mental performance [1]. However, EN consumption, especially in large quantities, is associated with cardiovascular events such as cardiac arrhythmias, chest pain, hypertension, and even sudden cardiac death [2]. Thus, the safety of long-term use of EN is questionable.The explosive growth in EN consumption in recent years has prompted the scientific community to investigate the impact of such products on human health and identify the reasons for their consumption. In addition, in recent years there has been an increase in the incidence of side effects associated with the use of EN, with the most common side effects being nervous, cardiovascular and gastrointestinal failure.The risks are even higher if EN is combined with alcohol. Several studies have examined the effects of EN in combination with alcohol and have shown that EN significantly reduce the immediate effects of ethyl intoxication, which may lead to increased consumption of alcoholic beverages [3]. Ferreira et al [4] showed that when combined with alcohol, energy drinks reduce the feeling of intoxication.Overall, the short- and long-term effects of EN consumption are inconsistent. Several studies have examined the short-term effects of EN by targeting specific components of EN rather than EN in general, and have reported positive short-term effects caused by these specific components. For example, caffeine has been shown to activate AMP-activated protein kinase (AMPK), which is a key enzyme coordinating multiple signaling pathways involved in maintaining cellular energy homeostasis [5]. In addition, caffeine has sympathomimetic effects, increasing cardiac activity and blood pressure [6].It has also been proven that taurine is an indirect regulator of oxidative stress in the myocardium, stabilizing cell membranes through direct interaction with phospholipids. It exhibits various biological activities such as positive effects on calcium kinetics as well as protection of cardiac function and is a modulator of protein kinases and phosphatases in cardiac muscle. In addition, it supports normal contractile function of the heart muscle [7] and lowers blood pressure [8].Some EN (ENERGY, Monster, Rockstar Energy and Red Bull 5-hour doses) contain niacin (vitamin B3) in doses exceeding the recommended daily intake. Niacin has a positive effect on restoring a healthy lipid profile and slowing the progression of atherosclerosis [9]. It has been used for more than half a century to treat lipid disorders such as abnormally elevated concentrations of LDL, triglycerides, and lipoproteins and low concentrations of HDL [9,10].In fact, most studies have shown that moderate consumption of EN over a short period of time improves cognitive and psychomotor performance [11]. However, some studies have also found the occurrence of negative effects from ED use, such as increased heart rate, systolic and diastolic blood pressure, and decreased cerebral blood flow [12,13].Finally, the long-term effects of EN consumption on various systems have not been well studied, and the effects of such drinks on the cardiovascular system are unknown. However, based on known information (see previous paragraph), our hypothesis is that EN consumption represents a significant risk factor for cardiovascular disease. The purpose of this study was to study the effects of chronic use of EN (Tornado), as well as to identify the effects on certain biochemical parameters and myocardial ultrastructure.
2. Methods
- ReagentsAll reagents used in this study were of analytical grade and were purchased from Fortek, Uzbekistan. EN Tornado was purchased at the local market.AnimalsThe study was conducted on male Wistar rats, which were kept under standard conditions and had free access to water and food.Twenty male Wistar rats weighing 179.6±4.3 g were divided into the following two groups of ten animals each: control (C) and Tornado (T). All animals received a standard diet. Group C had free access to tap water, Group T received 1.5 ml/100 g body weight of Tornado orally in drinking water daily for 30 days.In the last 6 days of the experiment, the animals were tested for physical performance by performing a forced swimming test with weights. The animals were forced to swim until exhaustion with 10% of their body weight attached to their tails. Each rat was considered to have reached exhaustion if it remained underwater for ≥5 s. Water temperatures ranged from 28°C to 30°C, and none of the animals exhibited hypothermia.After 30 days of treatment, immediately after exhaustion, the animals were sacrificed under anesthesia. Serum and cardiac muscle samples were collected for biochemical and ultrastructural analyses, including measurements of total cardiac glucose, glycogen, cholesterol, and protein concentrations; in addition, ALT and AST activities in serum and cardiac muscle were measured.Biochemical testsTotal glucose concentration was determined using the Somogy Nelson colorimetric assay [14,15]. Glycogen concentration was determined by the Montgomery method [16] modified by Lo et al. [17]. Total cholesterol was determined using ferric chloride [18]. Total protein concentration was determined using the Bradford colorimetric assay [19] with “ready-to-use” Bradford reagent. AST and ALT activities were analyzed using the photocolorimetric assay of Reitman and Frankel [20].Ultrastructural analysisFor electron microscopic analysis, myocardial samples were fixed in 2.7% glutaraldehyde and 2% osmium tetroxide, washed successively in phosphate buffer and dehydrated in increasing concentrations of acetone. They were then embedded in Epon and sectioned at 50–90 nm using a Leica UC6 Ultra microtome on a glass knife. Sections were counterstained and imaged at 80 kV using TEM JEOL JEM-1010. Images were acquired with a Mega Wiew III camera [21].Statistical analysisAll data are presented as mean ± standard deviation (SD). To statistically analyze the effects of different treatments, one-way analysis of variance was performed using Dunnett's test. All data were analyzed using GraphPad Prism version 6 for Windows. Significance was considered at p values <0.05.
3. Results
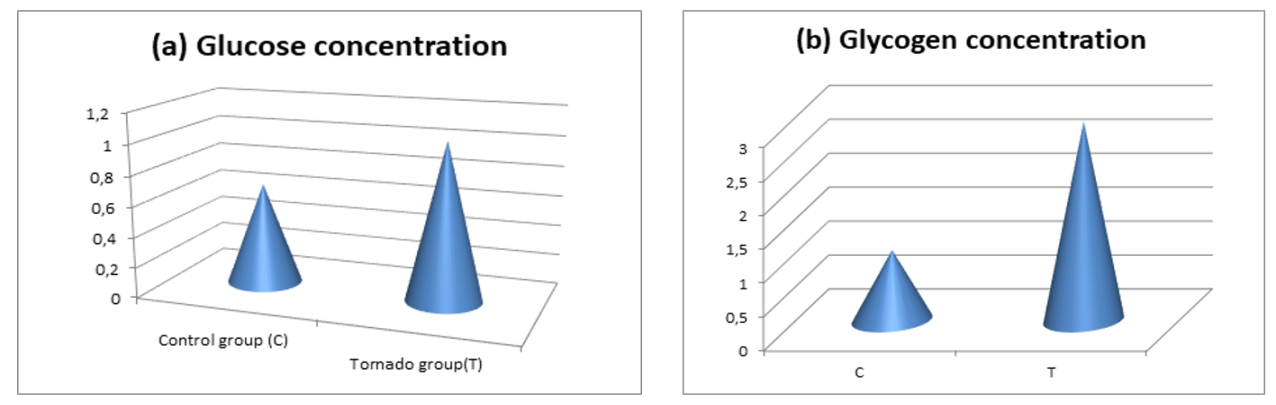
- Biochemical resultsThe effect of Tornado on the concentration of glucose and glycogen in the cardiac muscle is shown in Figure 1. These results showed that the glucose concentration increased in both groups, but more significantly in the T group (T 1.039 ± 0.1971 vs. C, 0.6699 ± 0.1467, p=0.0217*) (Fig. 1a). A clear increase in glycogen concentration (Fig. 1b) occurred in the Tornado group (T 2.932 ± 1.18 vs. C, 1.034 ± 0.3121; p = 0.0019**).
4. Discussion
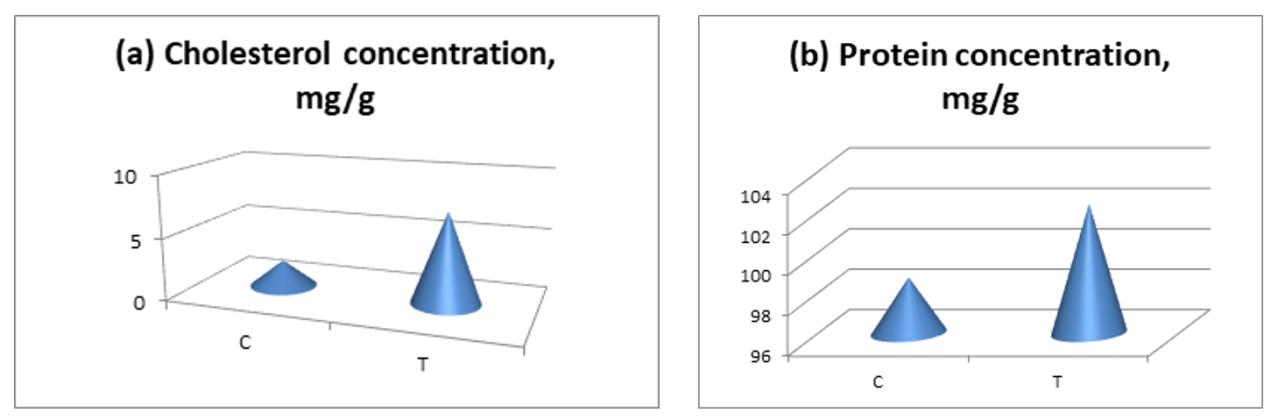
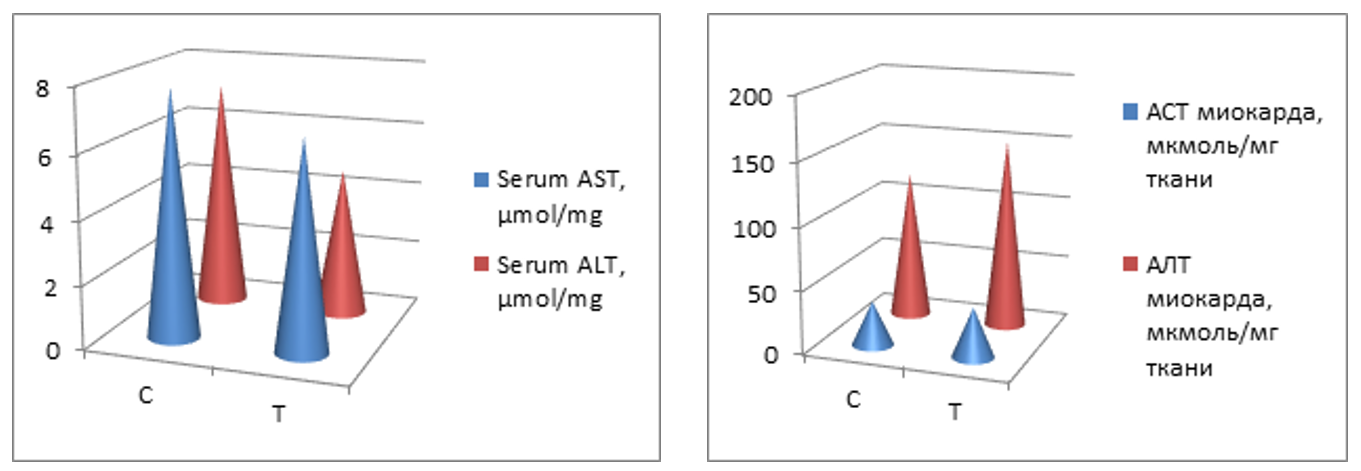
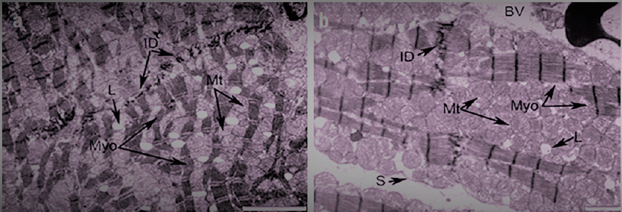
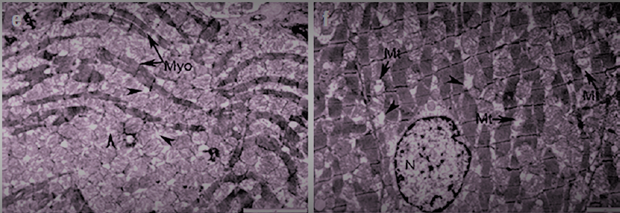
- This study shows for the first time that long-term consumption of EN causes biochemical and ultrastructural changes in cardiac muscle.Our results showed that Tornado increased myocardial glucose and glycogen concentrations. In the Tornado group, increases in glucose and glycogen concentrations were caused by two EN ingredients, caffeine and taurine. Normally, caffeine causes the release of calcium from intracytoplasmic stores [2] and activates AMPK via calcium/calmodulin-dependent protein kinase-β (CaMKK) [5]. AMPK promotes glucose uptake and utilization by cardiomyocytes [24]. In addition, AMPK either inhibits glycogen synthesis through phosphorylation of glycogen synthase or activates glycogen degradation through phosphorylation of glycogen phosphorylase [14]. However, chronic activation of AMPK, as likely occurred in our study, can increase glycogen synthesis by increasing glucose uptake and glucose-6-phosphate formation. This induces allosteric activation of glycogen synthase, which can overcome inhibitory phosphorylation by AMPK [11]. Additionally, taurine has been reported to increase glucose uptake, glycolysis, and glycogen synthesis in the heart of adult rats [23].In our study, ethanol led to a slight increase in glucose and glycogen concentrations. Ethanol reduces insulin sensitivity, which is mediated in cardiac muscle by tumor necrosis factor-α (TNFα) and/or interleukin-6 (IL-6) inducing activation of Jun N-terminal kinases, which inhibits the Akt-AS160-GLUT4 signaling pathway [15]. Thus, the glucose concentration should have decreased. We cannot explain these results, and there are no studies on this topic in the literature. However, the ultrastructural changes shown in Fig. 4 allowed us to assume that the Krebs cycle was not functioning correctly and, therefore, glucose metabolism in the myocardium was impaired.Glycogen is a vital molecule for normal myocardial function. It is essential for the ontogenetic development of the heart, as it provides the necessary energy for the growth and development of the organ [8]. In a mature organ, glycogen is contained in small quantities. Large amounts of glycogen are only beneficial under ischemic conditions [20]. The accumulation of glycogen in the myocardium contributes to the development of pre-excitation syndrome [7]. Several studies have reported an association of EN with adverse cardiovascular effects (palpitations, cardiac arrhythmias, hypertension, and even sudden cardiac death) [2,13]; therefore, the possibility that these effects were due to glycogen accumulation in the myocardium cannot be excluded.Cholesterol concentrations significantly decreased in all groups. This effect, in turn, may be responsible for the myocardial dysfunction reported with chronic EN use. The role of cholesterol is to strengthen cell membranes and maintain cell shape by forming “bridges” (lipid rafts) in areas where membrane proteins are expressed [25]. In addition, cholesterol controls membrane fluidity and therefore plays an important role in the cholesterol-to-phospholipid ratio [1]. The molar ratio of cholesterol to phospholipids in plasma membranes is typically maintained just below unity [18]. Therefore, a decrease in cholesterol concentration can lead to membrane destabilization, which in turn can affect cellular metabolism in the myocardium.The reduction in cholesterol concentrations caused by Tornado could be due to the increased taurine and/or niacin content of EN. This change is somewhat expected, since both taurine and niacin are used to prevent and treat atherosclerosis [4,12]. More specifically, taurine reduces serum cholesterol [10], and niacin reduces serum cholesterol and triglycerides and increases HDL concentrations [14].The formation of protein bonds may be an explanation for the significant increase in protein concentration in the T group observed in our study. Studies have shown that EN causes the accumulation of protein bonds in liver, nerve, and muscle tissues, exacerbating Tornado-induced toxicity in these tissues [9]. In a study by Worrall et al. [28], increased amounts of reduced acetaldehyde, unreduced acetaldehyde, and malondialdehyde-acetaldehyde protein adducts were found in rat cardiac tissue after 6 weeks of EN feeding. In addition, a previous study showed that adducts formed by acetaldehyde with proteins stimulate the formation of mRNA responsible for collagen synthesis and the expression of connective tissue proteins [17].The activity of AST and ALT increased in the myocardium and decreased in the serum after all types of exposure. Consistent with the ultrastructural changes induced by Tornado, the serum activity of these membrane integrity markers was expected to be increased. However, our results are consistent with those of Mihailovic et al. [25], who reported an increase in AST activity with unchanged ALT activity in cardiac muscle after 10 days of EN exposure. Our results indicate that Tornado has detrimental effects, at least on the heart muscle.Severe ultrastructural changes observed in the heart tissue of Tornado-treated rats (Figure 4) indicate the onset of cardiomyopathy. As noted by De Leiris et al. [21], humans and animal models exposed to chronic EN consumption undergo functional and structural changes in cardiac tissue. Oxidative stress causes lipid peroxidation, protein oxidation, impairing the contractility of the heart muscle. The organelles also show modified structures with disorganized cristae, leading to altered oxidative metabolism. Interestingly, we also found that the population of subsarcolemmal mitochondria was reduced while numerous areas of lysis were present.In some group T myocytes, the arrangement of myofibrils had a loose structure, and the space between them was occupied by several large (swollen) mitochondria with a sparse matrix and expanded cristae, which suggested a violation of oxidative metabolism. All of these morphological changes correlated with measured biochemical changes in glucose, glycogen and cholesterol concentrations, as well as AST and ALT activities.
5. Limitations of the Study
- Our experimental groups were relatively small, but allowed for statistical processing of the results. In addition, the duration of further experiments should be increased to better understand the long-term effects of EN use and to identify possible mechanisms of adaptation to their components. In addition, we used only one ED. Therefore, we believe that more research is needed using several of these drinks, especially since they have different compositions.
6. Conclusions
- Our results go some way to explaining the symptoms reported in the literature among those who use EN in large quantities or over a long period of time. Specifically, we are referring to the accumulation of glycogen in the myocardium, which can impair cardiac function and contribute to tachycardia, palpitations, cardiac arrhythmias, hypertension, and even death [2]. Decreased cholesterol concentrations, in turn, may be responsible for the myocardial dysfunction reported in the literature after chronic EN use. Our results showed that ENs induce morphological changes in cardiac muscle similar to those induced by ethanol. Further research is needed on various EDs in general and individual components to deeply understand their harmful effects and the mechanisms by which they are produced.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML