Sayitkhanov Saidanvarkhan
Tashkent State Dental Institute, Tashkent, Uzbekistan
Correspondence to: Sayitkhanov Saidanvarkhan, Tashkent State Dental Institute, Tashkent, Uzbekistan.
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The article is devoted to the changes of electrolyte imbalance in different stages of chronic kidney disease due to hyperhomocysteinemia. These indicators such as dyselectrolytemia and hyperhomocysteinemia were studied in 112 patients in research groups.
Keywords:
Potassium, Sodium, Calcium, Phosphorus, Homocysteine, Chronic kidney disease
Cite this paper: Sayitkhanov Saidanvarkhan, Hypergomocysteinemia-Dependent Dyselectrolithemia at Various Stages of Chronic Kidney Disease, American Journal of Medicine and Medical Sciences, Vol. 14 No. 5, 2024, pp. 1145-1150. doi: 10.5923/j.ajmms.20241405.01.
1. Introduction
Chronic kidney disease has become a global epidemic by the 21st century, and today its prevalence is 14% in the United States and 5-15% worldwide [10,21]. Because the kidneys play a key role in regulating fluid, electrolyte, and acid-base balance in the body, many water-electrolyte disturbances such as hyperkalemia, metabolic acidosis, and hyperphosphatemia occur in CKD and terminal CKD. This, in turn, leads to serious complications such as muscle atrophy, bone resorption and mineral exchange disorders, and vascular calcification, ultimately leading to death [3]. Although it is possible to coordinate some disorders with kidney transplantation in some patients of the last stage of CKD, however, hemodialysis, which is currently performed for most of these patients, does not fully solve the problem, that is, it is far from ideal [3]. Here, the most important and dangerous indicator of potassium content in the pathogenesis of CKD is an important gradient in the development of the disease, as well as in the occurrence of CKD pathologies and in determining the risk factor of CKD death in CKD patients. In the body, potassium is more than 98% intracellular and less than 2% extracellular. Disorders of potassium balance in CKD and especially in the terminal stage of CKD lead to an increase in the risk of inevitable CKD pathologies and death [16].Also, the pathogenetic mechanisms of CKD, the factors causing its development, exacerbation, diagnosis and treatment-prophylaxis issues are always in the focus of the world's leading scientists. In CKD patients, often the development of complications of CKD and its ending with lethality make the issue even more serious [4,6,20]. Systemic or glomerular hypertension, proteinuria, cytokines, hypoxia, impaired glomerular filtration, changes in podocytes, and disorders of endothelial cell production are risk factors for the development of CKD [8,12]. At the same time, homocysteine (Hcy) is a factor that increases the risk of CKD complications with renal dysfunction [4,9,19].In general, the imbalance of water-electrolyte balance is a large scale in the pathogenetic chain of diseases with CKD or kidney failure. The conducted research and obtained results are insufficient to provide adequate diagnosis and treatment of CKD patients. Therefore, it is necessary to study, compare, and apply new methods for diagnosis of changes in water-electrolyte and other endogenous substance (Hcy) factors in patients with CKD at different stages of the disease. The section of nephrology and hemodialysis requires the development of new recommendations and guidelines for optimal maintenance of water-electrolyte balance in the body in case of kidney dysfunction based on the evaluation of its indicators and the analysis of the obtained results.The purpose of the study. Analysis of the dynamics of changes in electrolyte balance disorders in relation to hyperhomocysteinemia in different stages of chronic kidney disease. Assessment of the risk of dyselectrolytemia and hyperhomocysteinemia in patients with exacerbation of renal failure.
2. Materials and Methods
87 patients with the 3rd and 4th stages of CKD treated in the nephrology department of the multidisciplinary clinic of the Tashkent Medical Academy were separated and two research groups were formed from them. 1st group consisted of 53 CKD stage 3 patients, 2nd group consisted of 34 CKD stage 4 patients. Also, the 3rd group (n-25) was formed from patients with 5th stage of CKD, who were receiving scheduled hemodialysis sessions at Republican Specialized Scientific And Practical Medical Center of Nephrology and Kidney Transplantation. The average age of patients in 1st group is 34.9±5.73 years, the average duration of the disease is 5.56±2.04 years; In 2nd group, the average age of patients is 39.3+10.8 years, the average duration of the disease is 7.31±2.13 years; The average age of patients in 3rd group is 41.6+8.63 years, the average duration of hemodialysis is 4.78±2.37 years. Potassium, sodium, calcium, phosphorus and homocysteine were checked in the blood serum of all patients by a special immunoenzyme analysis method. In order to ensure the randomization of the research group, the analysis samples were taken from the 3rd group, i.e. scheduled hemodialysis patients, on the day of starting the next hemodialysis session, before extracorporeal therapy. The obtained results were statistically analyzed.
3. Discussion of Results
The following results were obtained in the investigations carried out in the research groups. Accordingly, potassium was 4.47±0.24 μmol/l in 1st group, consisting of patients of the conservative stage of CKD; 5.6±0.32 and 5.9±0.26 μmol/l in 2nd group consisting of pre-dialysis patients and 5.9±0.26 μmol/l in 3rd group consisting of terminal stage patients. When the results were compared to the control group, it was reflected in the statistical analysis that the amount of potassium in the 1st group did not change reliably, and in the 2nd and 3rd groups, the values increased reliably (p<0.01, p<0.001). Also, when the groups consisting of patients of the late stages of CKD were statistically analyzed compared to 1st group, it was observed that potassium increased reliably in 2nd group (p<0.01) and reliably (p<0.001) in 3rd group, which caused mild hyperkalemia at this stage of the disease (Table 1).Sodium decreased to 139.2±1.71 μmol/l in 1st group, 151.7±1.93 in 2nd group, and 158.9±1.87 μmol/l in 3rd group. When the results were compared to the control group, it was shown in the statistical analysis that in 1st group sodium decreased less reliably (p<0.05), in 2nd group less reliably (p<0.05) and in groups 3 it increased this electrolyte reliably (p<0.001). Also, when the results were statistically analyzed compared to 1st group, sodium increased reliably in 2nd group (p<0.01) and reliably (p<0.001) in 3rd group, and even mild hypernatremia was noted in 3rd group (Table 1).Table 1. Dynamics of serum electrolytes and homocysteine changes in different stages of renal failure
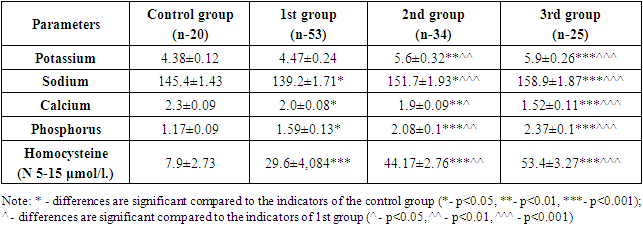 |
| |
|
Calcium decreased to 2.0±0.08 μmol/l in 1st group, 1.9±0.09 in 2nd group, and 1.52±0.11 μmol/l in 3rd group. When the results were compared to the control group, calcium was less reliable (p<0.05) in 1st group, more reliable (p<0.01) in 2nd group, and more reliable (p<0.001) in 3rd group patients, not only in exact numbers, but also in it was also confirmed in reliable statistical analyses. Also, when the results were statistically analyzed compared to 1st group, it was found that calcium in 2nd group was less reliable (p<0.05) and 3rd group was more reliable (p<0.001), causing significant hypocalcemia in the body (Table 1).It was found that phosphorus increased to 1.59±0.13 μmol/l in 1st group, 2.08±0.1 in 2nd group, and 2.37±0.1 μmol/l in 3rd group. When the results were compared with the control group, it was shown in the statistical analysis that phosphorus was less reliable (p<0.05) in 1st group, reliable (p<0.001) in 2nd group and 3 patients. Also, when the results were statistically analyzed compared to 1st group, it was observed that phosphorus increased reliably in 2nd group (p<0.01) and reliably (p<0.001) in 3rd group, causing hyperphosphatemia in blood serum (Table 1).If we look at the images of the diagram formed on the basis of indicators of electrolyte imbalance in different stages of kidney failure, we can see that the process is progressing in a negative direction. Accordingly, the decrease in potassium in the G 2 stage compared to the control group, although uncertain, is explained by polyuria in this stage. Although polyuria persists even in the G 3 stage, a sharp decrease in GFR indicates a mild degree of hyperkalemia in patients at this stage. This risk was more pronounced in the study groups of CKD stage 5 patients with hyperkalemia moving towards moderate severity (Figure 1).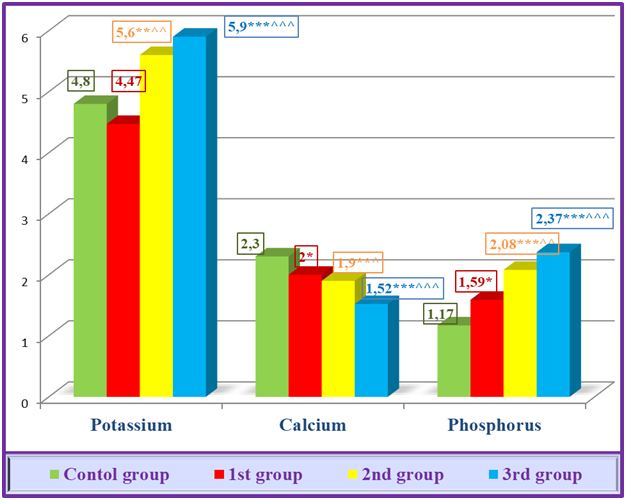 | Figure 1. Electrolyte dynamics at the different stages of CKD |
Hyperkalemia is a common dangerous consequence of end-stage renal failure [13]. First of all, if renal dysfunction causes hyperkalemia, a number of other conditions, including slowing of transcellular transport due to insulin deficiency, mineral metabolic acidosis (hemolysis (typical for extracorporeal therapy), rhabdomyolysis, lysis of ischemic tissues and tumors), high consumption of potassium (especially in CKD patients), medication (ACE and aldosterone inhibitors, angiotensin receptor blockers, mineralocorticoid receptor antagonists, calcineurin inhibitors) also cause hyperkalemia. [2]. Another complicating factor is that hyperkalemia precludes recommending many life-saving drugs for CKD patients. RAAS inhibitors are a good example of this. In the study of the database of CKD patients treated with RAAS inhibitors (n=205), it was found that RAAS inhibitors were stopped in 16-18% of patients due to the hyperkalemia that developed due to these drugs. However, it is even more unfortunate that among those who stopped taking RAAS inhibitors, the death rate was three times higher than among those who continued treatment [11].Calcium and phosphorus balance also reflected a unique picture. If CKD progresses, serum calcium decreases and phosphorus content increases (Fig. 1). Importantly, the occurrence of these changes in the early stages of kidney failure, that is, in the conservative stage, was once again confirmed during the statistical analysis.The fact that sodium in the diagram is less reliable (p<0.05) in 1st group compared to the control group is explained by polyuria in the conservative stage of CKD. In literature in general his GFR averages 50 ml/min. of a cohort of 655,000 CKD patients, hyponatremia was observed in 13.5% and hypernatremia in 2% [15]. 2nd group, i.e., in the pre-dialysis stage, sodium is maintained in the norm, but here, despite the preservation of polyuria, the risk of hypernatremia is approaching due to the sudden decrease in GFR. This is evident by the increase in sodium value, less reliable than the control group (p<0.05) and more reliable than the 1st group (p<0.001). The occurrence of hypernatremia in patients of the terminal stage, i.e., 3rd group, was clearly reflected not only in the statistical analysis, but also in the diagrams formed based on these electrolyte indicators (Fig. 2).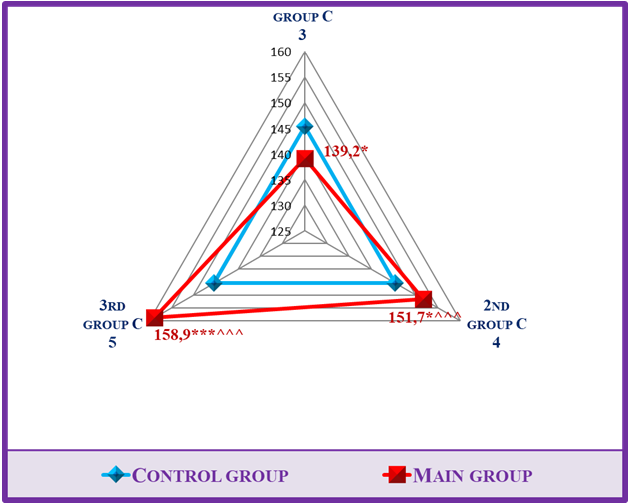 | Figure 2. Electrolyte dynamics at the different stages of CKD |
If hypernatremia persists for a long time, it is mainly observed in people who have impaired thirst mechanism or are unable to drink water. Dysnatremia is important for mortality in CKD and CKD. U-shaped relationship between serum sodium and mortality was found in pre-dialysis CKD and dialysis patients. In this case, it is necessary to revise the scheduled hemodialysis program for dialysis patients and make corrections to it [17,18].Homocysteine- this is a sulfur-containing amino acid, which is not part of the protein, and is an intermediate product of important regulatory processes transmethylated in all cells [14]. Cysteine (Cys) is considered to be the precursor of homocysteine, which in turn is the precursor of glutathione, the main endogenous antioxidant of mammalian cells, including humans [16]. The amount of Hcy in the blood serum of a healthy person is 5.0-7.0 μmol/l. will be in the range. According to the American Association of Cardiology, the concentration of Hcy in humans is 10.0 μmol/l. reaching the limit is caused by the following risk factors: intestinal malabsorption syndrome, hypothyroidism, kidney failure, family anamnesis occurs in persons with congenital CKD diseases [7]. In our studies, the dynamics of homocysteine reflected the landscape of changes in sheep. According to him, in 1st group, consisting of patients in the early stages of renal failure (S 2-3), homocysteinelevel 29.6±4,084μmol/l; in 2nd group consisting of pre-dialysis patients (S 4). 44.17±2.76 mamol/l. and 53.4±3.27 μmol/l in the 3rd group of scheduled hemodialysis patients. it was determined that When the results were compared to the control group, a reliable (p<0.001) increase in homocysteine in all study groups was proven in statistical analysis. Also, when the results were statistically analyzed compared to 1st group, this indicator increased reliably in 2nd group (p<0.01) and reliably (p<0.001) in 3rd group, which indicates the progressive development of hyperhomocysteinemia (Table 1).The diagram shows that mild hyperhomocysteinemia occurs in CKD patients from the early stages of renal failure. Also, in the late stages and hyperhomocysteinemia of moderate severity occurs. This was confirmed not only in the exact arithmetic results of the laboratory tests, but also in the confirmation of the reliable (p<0.001) change of the values in the statistical analyzes (Fig. 3).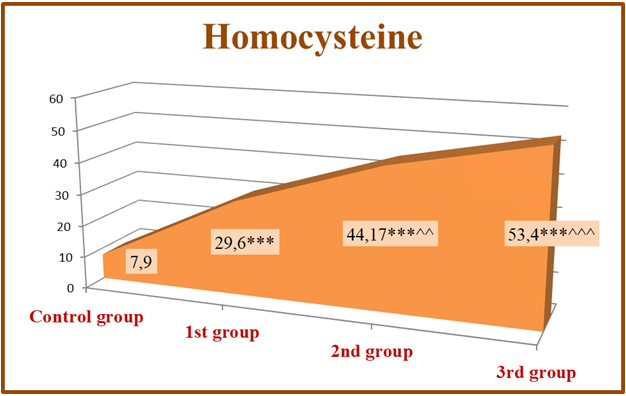 | Figure 3. Hyperhomocysteinemia in different stages of CKD |
These changes can be seen in the literature notes of scientists who have conducted research in this regard. Including hyperhomocysteinemiadue to a decrease in the clearance of homocysteine through the kidney in the case of kidney dysfunction, it has a negative effect on the vascular intima, increases dyslipidemia and causes hypercoagulation, which is a very serious risk factor for the development of cardiovascular diseases in CKD patients [5]. Also, ghyperhomocysteinemia definitely occurs in patients with CKD, usually it is evident in the pre-dialysis and terminal stages of kidney failure [1]. Therefore, the concentration of Hcy in the serum will be specific in different stages of CKD. Hcy begins to rise in the serum from the early stages of renal failure [7]. In CKD patients, the maximum level of Hcy occurs during the dialysis period, in which the damage and complications of the CKD are evident. According to the team of authors [7], hyperhomocysteinemia occurs in 40% of patients in the early stages of CKD, in 90% in the pre-dialysis stage, and in 94.7% of dialysis patients. This pathological process is especially dangerous for patients with a lack of CVS, in whom the risk of death is recognized to be 30 times higher compared to the main group of patients with CVS. Clearly, Hcy concentration increases in renal failure and it exhibits moderate hyperhomocysteinemia in dialysis patients (>85%). Also, serum Hcy in renal failure is inversely correlated with creatinine clearance, that is, with glomerular filtration [15]. When the GFR decreases from 90 ml/min to 60 ml/min, the amount of Hcy is doubled, and when it decreases from 60 ml/min, the concentration of Hcy in the serum increases by 9-11 times [4].At the same time, when we observed the relationship between serum homocysteine concentration and electrolytes in CKD patients, it was observed that potassium and phosphorus were correctly correlated, and calcium was weakly inversely correlated. The correlation wasn’t observed between sodium and homocysteine (Figure 4).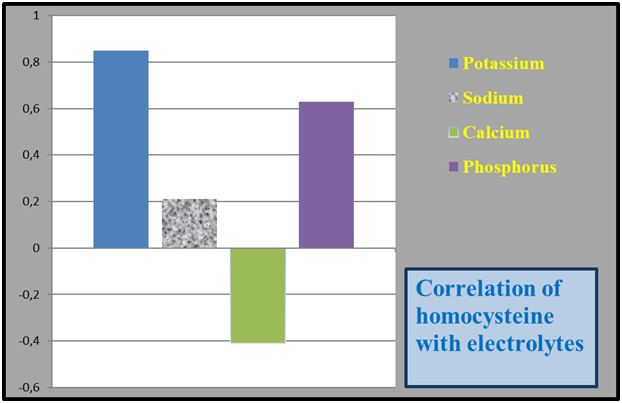 | Figure 4. Correlation of serum homocysteine levels with electrolytes in patients |
Thus, water-electrolyte imbalances, which are somewhat neglected, occur together with hyperhomocysteinemia in CKD patients, and they seriously affect a number of pathophysiological mechanisms in the cardiovascular system of patients, forming CHF. CHF, on the other hand, is inevitable because it is an undeniable ground for the further development and deepening of CKD. Therefore, the problem of controlling and correcting homocysteine level in blood serum from the early stage of CKD is one of the urgent problems facing specialists in the therapeutic field of clinical practice of nephrology.
4. Conclusions
1. At various stages of chronic kidney disease, the water-electrolyte balance changes in its own way, and it leads to a further decrease in glomerular filtration.2. As chronic kidney disease progresses from the conservative stage to the terminal stage, potassium and sodium levels increase to the level of hyperkalemia, and hypocalcemia and hyperphosphatemia are added to the process during this period.3. Hyperhomocysteinemia is mild in the conservative stage of chronic kidney disease, and moderate in the pre-dialysis and terminal stages.4. Hyperhomocysteinemia in chronic kidney disease, because it is a very serious risk factor for the development of cardiovascular diseases, it is necessary to monitor the level of homocysteine in the blood serum from the early stages of CKD.
References
| [1] | Aleksandrova L.A., Subbotina T.F., Zhloba A.A. The relationship between folate deficiency, hyperhomocysteinemia and glutathione metabolism in patients with arterial hypertension. Arterial hypertension. 2020; 26(6): 656–64. Doi: 10.18705/1607-419X-2020-26-6-656-664. |
| [2] | Belyalov FI. Cardiac arrhythmias. 8th edition, revised and expanded // Moscow. GEOTAR-Media. 2020. |
| [3] | Dhondup T. · Qian K. Electrolyte and acid-base disturbances in chronic kidney disease and end-stage renal failure // Blood Purif (2017) 43 (1-3): 179–188. |
| [4] | Konyukh E.A., Naumov A.V., Paramonova N.S. Homocysteine: role in the development and progression of chronic kidney disease // Nephrology. 2011. Volume 15. No. 3. pp. 18-25. |
| [5] | Murkamilov I.T., Aitbaev K.A., Fomin V.V. and others. Homocysteine and the risk of nephrocerebrovascular diseases. Sci. Heritage. 2020; 50(2): 29–35. |
| [6] | Murkamilov I.T., Sabirov I.S., Fomin V.V., Murkamilova Zh.A., Yusupov F.A. Electrolyte imbalance and cardiac arrhythmias in chronic kidney disease. // The scientific heritage No. 60 (2021) 55 C 55-70. |
| [7] | Murkamilov I.T., Aitbaev K.A., Fomin V.V., Murkamilova Zh.A., Yusupov F.A., Kudaibergenova I.O. Homocysteine and folic acid in chronic kidney disease: clinical and prognostic significance // Clinical Nephrology No. 3 / 2021. pp. 49-56. https://dx.doi.org/10.18565/nephrology.2021.3.49-56Al Yazeedi B. The Importance of Obesity as a Risk Factor for Hyperhomocysteinemia: An Overview. Nutr. Management Met. Asp. Hyperhomocysteinem. 2021. 173 с. |
| [8] | Cerqueira A., Quelhas-Santos J., Sampaio S., et al. Endothelial Dysfunction Is Associated with Cerebrovascular Events in Pre-Dialysis CKD Patients: A Prospective Study. Life. 2021; 11(2):128. Doi: 10.3390/life11020128. |
| [9] | De Nicola L, Zoccali C: Chronic kidney disease prevalence in the general population: heterogeneity and concerns. Nephrol Dial Transplant 2016; 31: 331-335. |
| [10] | Epstein M, et al: Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 2015; 21(11 suppl): S212-S220. |
| [11] | Fogo AB. Mechanisms of progression of chronic kidney disease. Pediatr Nephrol 2007; 22: 2011–2022. |
| [12] | Ingelfinger JR: A new era for the treatment of hyperkalemia? N Engl J Med 2015; 372: 275-277. |
| [13] | Kai W., Kun L., Lin X., et al. Mechanism of hyperhomocysteinemia induced renal injury in Cbs+/-mice. Chin. J. Tissue Engineer. Res. 2021; 25(11):1728–32. Doi: 10.3969/j.issn.2095-4344.3084. |
| [14] | Kovesdy CP, et al: Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation 2012; 125: 677-684. |
| [15] | Luo J, et al: Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol 2016; 11: 90-100. |
| [16] | Ravel VA, et al: Serum sodium and mortality in a national peritoneal dialysis cohort. Nephrol Dial Transplant 2016; pii: gfw254. |
| [17] | Rhee CM, et al: Pre-dialysis serum sodium and mortality in a national incident hemodialysis cohort. Nephrol Dial Transplant 2016; 31: 992-1001. |
| [18] | Shishehbor MH, Oliveira LP, Lauer MS, et al. Emerging cardiovascular risk factors that account for a significant portion of attributable mortality risk in chronic kidney disease. Am J Cardiol 2008; 101: 1741–1746. |
| [19] | Suresh S., Waly M.I. Metabolic Role of Hyperhomocysteinemia in the Etiology of Chronic Diseases. Nutr. Management Met. Asp. Hyperhomocysteinem. 2021. 51 с. |
| [20] | United States Renal Data System: 2015 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, National Institutes of Health, 2015. www.usrds.org/2015/view/Default.aspx. |






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML