Ibragimov Kh. I., Sultonov I. I., Ziyadullayev Sh. X.
Department of Internal Medicine 1, Samarkand State Medical University, Samarkand, Uzbekistan
Correspondence to: Sultonov I. I., Department of Internal Medicine 1, Samarkand State Medical University, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This observational cohort study assessed the efficacy and safety of adalimumab and methotrexate combination therapy in patients with rheumatoid arthritis (RA) who had an inadequate response to methotrexate monotherapy. Patients were divided into three groups: Group IA received adalimumab 40 mg biweekly plus methotrexate 7.5 mg weekly, Group IB received adalimumab 40 mg biweekly plus methotrexate 15 mg weekly, and the comparison group received methotrexate 15 mg weekly. The primary outcome was the change in the Disease Activity Score based on 28 joints (DAS28) from baseline to 6 months. Secondary outcomes included the American College of Rheumatology (ACR) 20, 50, and 70 responses, changes in the Health Assessment Questionnaire-Disability Index (HAQ-DI) scores, and radiographic progression assessed using the modified Sharp/van der Heijde score (mTSS). Results showed that the combination of adalimumab and methotrexate was significantly superior to methotrexate monotherapy in achieving clinically significant responses (ACR20, ACR50, and ACR70) over nine months of treatment. Group IB, receiving a higher dose of methotrexate, exhibited slightly better clinical improvement rates than Group IA, although the difference was not statistically significant. By nine months, 88.9% of patients in Group IB achieved an ACR20 response, compared to 74.2% in Group IA and 46% in the comparison group. Similar trends were observed for ACR50 and ACR70 responses. The study suggests that the combination of adalimumab and methotrexate is clinically superior to methotrexate monotherapy in the treatment of RA, with a higher methotrexate dose potentially leading to better outcomes.
Keywords:
Rheumatoid arthritis, Adalimumab, Methotrexate, Observational study, Disease activity, ACR20, ACR50, ACR70
Cite this paper: Ibragimov Kh. I., Sultonov I. I., Ziyadullayev Sh. X., Clinical Effectiveness of Adalimumab and Methotrexate Combination Therapy in Rheumatoid Arthritis, American Journal of Medicine and Medical Sciences, Vol. 14 No. 4, 2024, pp. 1109-1115. doi: 10.5923/j.ajmms.20241404.64.
1. Introduction
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disease characterized by inflammation of the synovial joints, leading to progressive joint destruction, pain, and disability. The etiology of RA is complex and multifactorial, involving genetic, environmental, and immunological factors that contribute to the pathogenesis of the disease. The clinical manifestations of RA are diverse, ranging from mild joint stiffness to severe joint damage and systemic complications, impacting the quality of life of affected individuals [1-4,16].The management of RA has evolved significantly over the past few decades, with the advent of disease-modifying antirheumatic drugs (DMARDs) and biologic agents that target specific components of the immune system. Methotrexate is a conventional DMARD that has been a cornerstone in the treatment of RA due to its efficacy in controlling disease activity and slowing disease progression. However, not all patients respond adequately to methotrexate monotherapy, necessitating the use of combination therapy or alternative agents [1,4,6,9].Biologic agents, such as tumor necrosis factor (TNF) inhibitors, have revolutionized the treatment landscape of RA. Adalimumab, a fully human monoclonal antibody that inhibits TNF-α, has demonstrated significant efficacy in reducing the signs and symptoms of RA, inhibiting the progression of joint damage, and improving physical function when used alone or in combination with methotrexate [5,6].Despite the availability of effective therapies, the optimal treatment strategy for individual patients remains a challenge. The heterogeneity of the disease and the variability in patient response to treatment underscore the need for personalized approaches and the exploration of different treatment regimens. In this context, the present study aims to compare the efficacy and safety of different combinations of adalimumab and methotrexate in patients with RA who have an inadequate response to methotrexate monotherapy [7,8,12-15].This study is designed to provide insights into the comparative effectiveness of these treatment regimens, which may inform clinical decision-making and contribute to the optimization of therapeutic strategies for patients with RA. The results section of this article presents the findings of this investigation, including the demographic and baseline clinical characteristics of the patients, the impact of the treatment regimens on disease activity, and the safety profile of the combinations.
2. Materials and Methods
This observational cohort study was conducted to compare the efficacy and safety of different combinations of adalimumab and methotrexate in patients with rheumatoid arthritis (RA) who had an inadequate response to methotrexate monotherapy. Adult patients (aged ≥18 years) diagnosed with RA according to the 2010 American College of Rheumatology/European League Against Rheumatism criteria, with a disease duration of at least 6 months, were included in the study. Eligible participants had active disease, defined as having at least 6 swollen joints and 6 tender joints, along with either an erythrocyte sedimentation rate (ESR) >28 mm/hour or a C-reactive protein (CRP) level >1.0 mg/dL.Participants were categorized into three exposure groups based on their treatment regimen:Group IA: Adalimumab 40 mg subcutaneously every other week + methotrexate 7.5 mg orally once weeklyGroup IB: Adalimumab 40 mg subcutaneously every other week + methotrexate 15 mg orally once weeklyComparison Group: Methotrexate 15 mg orally once weeklyThe primary outcome was the change in the Disease Activity Score based on 28 joints (DAS28) from baseline to 6 months. Secondary outcomes included the American College of Rheumatology 20% improvement criteria (ACR20), ACR50, and ACR70 responses, changes in the Health Assessment Questionnaire-Disability Index (HAQ-DI) scores, and radiographic progression assessed using the modified Sharp/van der Heijde score (mTSS).Data on demographic characteristics, clinical parameters, treatment regimens, and outcomes were collected from medical records and patient interviews. Descriptive statistics were used to summarize baseline characteristics. Comparative analyses between the exposure groups were performed using chi-square tests for categorical variables and analysis of variance (ANOVA) or Kruskal-Wallis tests for continuous variables, as appropriate. Multivariable regression analyses were conducted to adjust for potential confounders and to estimate the adjusted effect of the treatment regimens on the outcomes.The study protocol was approved by the institutional review board or ethics committee at each participating center, and the study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent was obtained from all participants before enrollment in the study.
3. Results
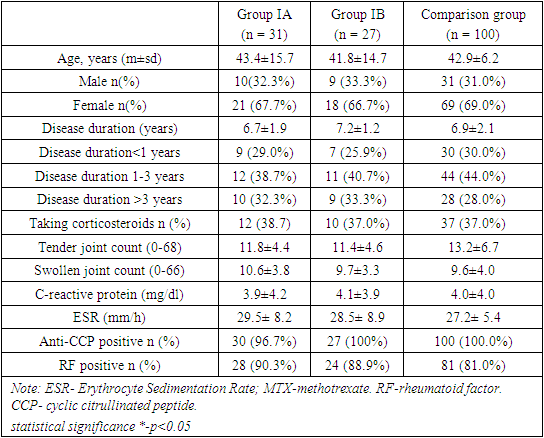
Demographic and baseline clinical characteristics of the patients reflected a population with RA and were comparable among the 3 treatment groups. We divided patients into three groups. Group - IA consisted of patients who received a combinations of adalimumab 40mg + methotrexate 7.5mg, IB- those who received a combinations of adalimumab 40mg + methotrexate 15mg and the comparison group received only methotrexate 15mg once weekly. The mean age of patients were 43.4±15.7 years in group IA, 41.8±14.7 years in group IB and 42.9±6.2 years of the patients following methotrexate (MTX) monotherapy in comparison group. We found no statistically significant difference between the comparison groups in terms of mean age of participants (p>0.05). Majority of the patients were females in all three groups with no difference in the female male ratio between the groups (67.7%, 66.7% and 69.0% respectively). In each treatment group, the mean duration of RA at baseline was 6.7, 7.2 and 6.9 years respectively. Majority of patients had disease duration of between 3-7 years. Of notes, 12 (38.7%) patients in IA group, 11 (40.7%) patients in IB group and 44 (44.0%) patients taking methotrexate monotherapy had disease duration of between 3 to 7 years. Moreover, over 60% of the study patients in all groups had RA for over 5 years. We found no statistically significant difference between the comparison groups in the disease duration (p>0.05). Similar percentages of patients in each treatment group had previously received treatment with a DMARD (other than MTX). Among all patients who previously received DMARDs, 41% had received leflunomide and 39% had received sulfasalazine. Approximately one-third of patients in each treatment group were taking corticosteroids at baseline (38.7%, 37.0% and 37.0% respectively). The mean corticosteroid dosage (prednisone equivalent) was 12.7 mg/day in the IA treatment arm, 13.1 mg/day in the IB treatment arm, and 14.8 mg/day in the comparison group (table 1). Table 1. Characteristics of patients according to treatment group
 |
| |
|
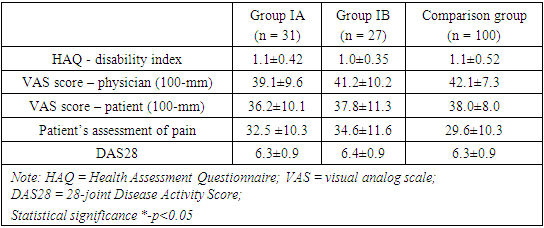
In terms of joint involvement, both tender and swollen joint counts exhibited similar values across Group IA (11.8±4.4 and 11.4±4.6, respectively), Group IB (10.6±3.8 and 9.7±3.3, respectively), and the comparison group (13.2±6.7 and 9.6±4.0, respectively). These figures imply a consistency in the severity of joint tenderness and swelling at the baseline within this patient cohorts. Similarly, the levels of inflammatory markers, CRP (Group IA: 3.9±4.2, Group IB: 4.1±3.9, Comparison group: 4.0±4.0) and ESR (Group IA: 29.5±8.2, Group IB: 28.5±8.9, Comparison group: 27.2±5.4), did not display significant variations, indicating uniform disease activity among the groups at the baseline.Furthermore, the prevalence of anti-CCP positivity was notably high in all groups, with percentages of 96.7% in Group IA, 100% in Group IB, and 100.0% in the Comparison group. Similarly, RF positivity was prevalent, with percentages of 90.3% in Group IA, 88.9% in Group IB, and 81.0% in the Comparison group. These numbers underscore a consistent immunological profile among patients with RA at the commencement of the study. The lack of significant differences in the percentages of positive patients emphasizes the uniformity of autoimmune characteristics in the studied populations.The presented baseline characteristics elucidate a remarkable homogeneity among patients with rheumatoid arthritis in terms of joint involvement, inflammatory markers, and autoimmune markers. These actual numerical findings provide a concrete understanding of the initial disease presentation and lay the groundwork for further exploration of treatment responses and disease progression in these distinct patient groups (table 2).Table 2. Characteristics of disease activity and disability indexes in patients with RA
 |
| |
|
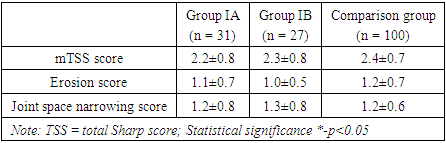
The mean HAQ disability index was 1.1±0.42 in group IA, 1.0±0.35 in group IB and 1.1±0.52 in the comparison group among those who received only methotrexate. There were not any statistically significant baseline differences among treatment groups in the HAQ disability score (p>0.05). The mean VAS score assessed by physician’s global assessment of disease activity did not show statistically significant baseline differences among the comparing groups with VAS score of 39.1±9.6, 41.2±10.2 and 42.1±7.3 respectively (p>0.05). Similarly, the analysis of mean VAS score assessed by patient’s global assessment of disease activity did not show significant baseline differences among the comparing groups (p>0.05).Table 3 delineates the baseline radiographic findings in patients with rheumatoid arthritis (RA) across three distinct groups. In terms of the TSS score, the numeric values indicate that group IA has a mean score of 2.2±0.8, group IB has 2.3±0.8, and the comparison group has 2.4±0.7. The associated p-values (p1>0.05, p2>0.05) suggest that there were low to moderate level of joint damages across groups and are no statistically significant differences in the overall joint damage, as measured by the TSS scale between the groups.Further examination of the erosion score reveals similar trends. Group IA demonstrates an erosion score of 1.1±0.7, group IB has 1.0±0.5, and the comparison group has 1.2±0.7. The p-values indicate no significant distinctions in bone damage among the groups.Similarly, the joint space narrowing score demonstrates comparable values across the groups. Group IA has a score of 1.2±0.8, group IB has 1.3±0.8, and the comparison group has 1.2±0.6, with p-values denoting no statistically significant differences in cartilage loss levels.The baseline radiographic findings, elucidated by actual numerical values and supported by p-values, portray a consistency in joint damage, bone erosion, and cartilage loss among patients with RA in the studied groups (table 3).Table 3. Baseline radiographic findings in patients with rheumatoid arthritis
 |
| |
|
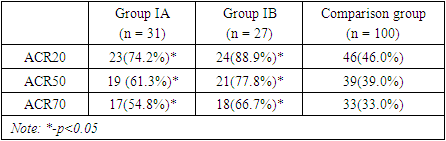
The table 4 represents the rate of clinically significant response to treatment defined as ACR20, ACR50 and ACR70 based on criteria given by American College of Rheumatology among three different treatment groups. The data shows that the combination of adalimumab and methotrexate was significantly superior to the methotrexate monotherapy over the period of 9 months of treatment in clinically significant response rate defined as 20%, 50% and 70% improvement from baseline. Table 4. Baseline characteristics of immunological indexes in patients with rheumatoid arthritis
 |
| |
|
The improvement rates in group IA was slightly lower than the rates among the IB group patients which suggests that combinations of adalimumab with 15mg of methotrexate may lead to better clinical improvement compared to the combination with 7.5mg of methotrexate, however, this trend was not statistically significant (p>0.05). By 9 months of treatment 88.9% of patients in IB group reached clinically significant 20% improvement compared to 74.2% patients in IA and only 46% in comparison group (p1<0.05, p2<0.05). Similar response rates trend was observed when assessed for clinically important 50% and 70% improvements in patients from baseline. As per results, by month 9, 66.7% of patients in group IB reached 70% improvement clinical, functional and laboratory findings compared to 54.8% patients in IA groups and only 33.0% of those receiving methotrexate monotherapy. The obtained results suggests that, adalimumab and methotrexate combinations is clinically significantly superior compared to methotrexate monotherapy in treatment of rheumatoid arthritis. The combination of adalimumab with 15 mg methotrexate tends to be superior to the combination with 7.5mg of methotrexate, however, statistically significant difference between combinations groups was not observed. The graph 4.1 presents a longitudinal comparison of the percentage of patients achieving a 20% improvement in symptoms, as defined by ACR20 criteria, over a period of nine months. From baseline to the 9-month mark, there is a notable increase in the percentage of patients achieving ACR20 across all groups. Group IA shows a consistent upward trajectory, starting at 22.0% at the first month and reaching 74.2% by the ninth month. Group IB, which received a higher dose of methotrexate in combination with adalimumab, starts at a similar level of improvement in the first month (26.0%) but shows a faster rate of increase, achieving the highest percentage of improvement across all groups by the end of the study period (88.9% at month 9). This suggests a dose-response effect, with higher doses of methotrexate potentially leading to better outcomes when combined with adalimumab (p<0.05). The comparison group, treated with methotrexate monotherapy, demonstrates a more gradual and less pronounced improvement ending at 46.0%. While there is improvement, it is considerably less than that seen in the combination therapy groups. Statistically, the graph indicates that both combination therapy groups (IA and IB) are significantly superior to the methotrexate monotherapy group, as evidenced by the final percentages and the apparent gaps between the curves. Specifically, Group IB's final ACR20 improvement rate is nearly twice that of the comparison group, which is clinically significant.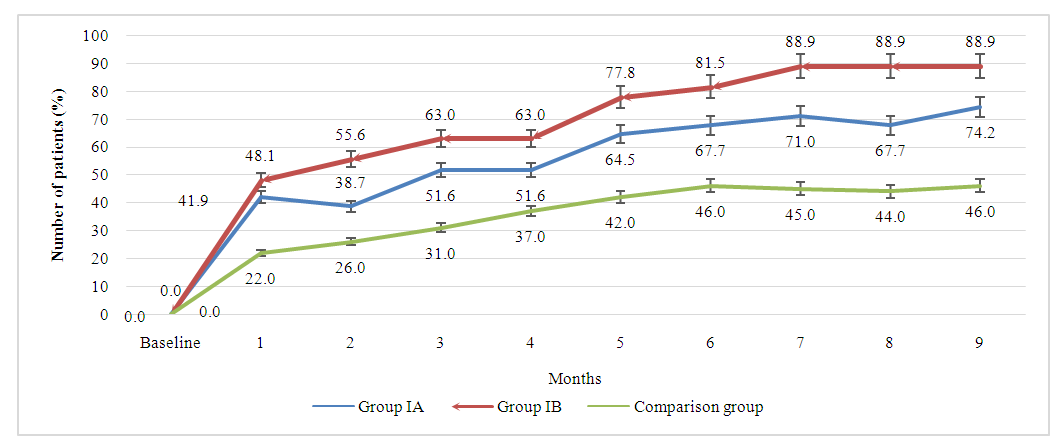 | Picture 4.1. The longitudinal comparison of response rate by ACR20 criteria across all treatment groups |
The graph 4.2 illustrates the progression of the percentage of patients achieving a 70% improvement in rheumatoid arthritis symptoms over a nine-month treatment period, as defined by ACR70 criteria. This higher threshold of improvement is indicative of a more substantial amelioration of symptoms. Over the nine months, group IA experiences a gradual increase in patient response, starting from 13.0% and reaching 54.8% by the ninth month. The upward trend is consistent, with occasional plateaus, reflecting a continuous yet variable response to the treatment. Group IB, on the other hand, demonstrates a more rapid initial increase, which suggests an early onset of significant improvement with the higher dose of methotrexate. After the initial surge, the increase continues at a steadier pace, eventually reaching 66.7% by month nine. This group consistently outperforms Group IA, indicating a dose-related response, where a higher methotrexate dose with adalimumab may be more effective. The comparison group, which received only methotrexate monotherapy, exhibits the most modest improvement throughout the study period with a final ACR70 response rate of 33.0% at nine months.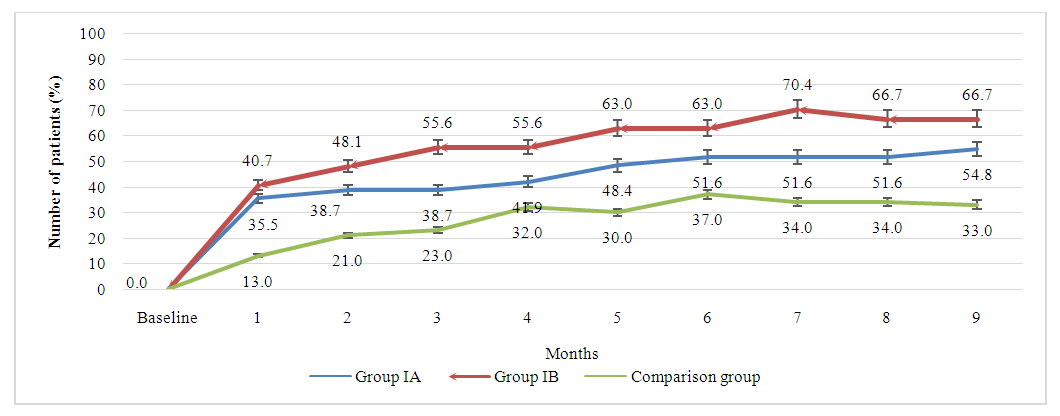 | Picture 4.2. The longitudinal comparison of response rate by ACR70 criteria across all treatment groups |
The graph 4.3 provides a detailed depiction of the changes in the number of tender joints among patients with rheumatoid arthritis over a nine-month period, segmented by combinations treatment groups IA, IB, and the comparison group. Over the course of the treatment, group IA showed a gradual decline in the mean number of tender joints, reaching 9.2 by the end of the ninth month. In comparision, group IB, treated with adalimumab in combination with a higher dose of methotrexate, exhibits a more pronounced decline in the number of tender joints. Starting from a similar baseline, the count drops sharply within the first month and continues to decline, ending at 4.2 tender joints by the ninth month. 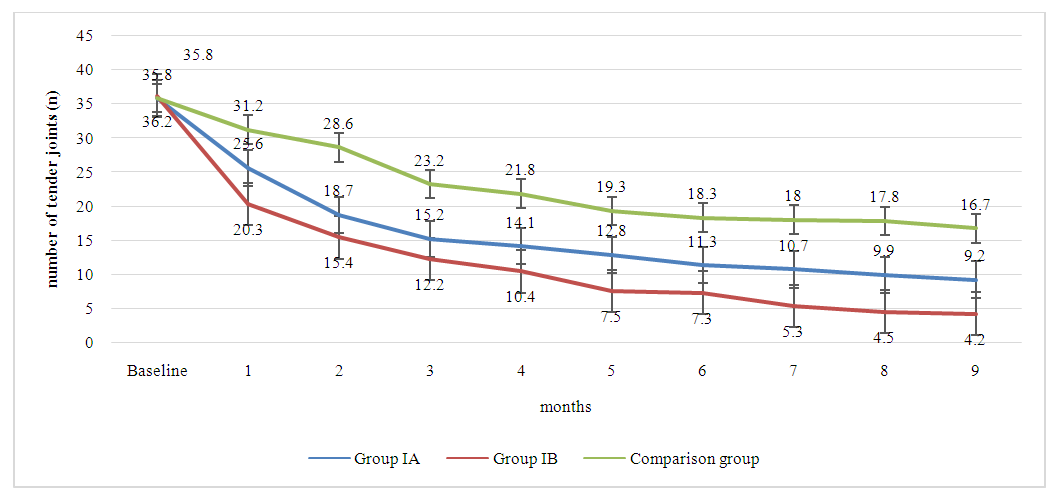 | Picture 4.3. The dynamics of tender joints count in patients with rheumatoid arthritis across all treatment groups |
This trend not only demonstrates the effectiveness of the treatment but also suggests that a higher dose of methotrexate may have a more potent effect on reducing joint tenderness when used alongside adalimumab. The comparison group, also showed a reduction in tender joints from the baseline. However, the decline is less steep compared to the combination therapy groups. By the ninth month, the comparison group's mean number of tender joints stands at 16.7, which, while indicative of improvement, highlights a less robust response to monotherapy versus combination therapy.The data suggests that while all treatments result in improvements in tender joints, the combination of adalimumab with methotrexate is more effective, and the efficacy increases with the methotrexate dosage. The graph effectively visualizes the superiority of combination therapy over monotherapy in the management of tender joints in rheumatoid arthritis patients. It underscores the importance of considering both the type and dosage of medication when treating this disease to achieve optimal patient outcomes.The graph 4.4 depicts the change in the number of swollen joints in patients with rheumatoid arthritis over a nine-month treatment period, segmented by treatment groups IA, IB, and the comparison group. Group IA, receiving a combination of adalimumab 40mg and methotrexate 7.5mg, demonstrates a steady decrease in swollen joint count from 27.9 to 8.6. This reduction suggests that the treatment is effective in reducing joint swelling, a key symptom and marker of inflammation in rheumatoid arthritis. Group IB, which was administered adalimumab 40mg in combination with a higher dose of methotrexate at 15mg, shows a more accelerated reduction in swollen joint count, starting at 29.2 and reaching 4.3 by the end of the nine months. The sharper decline relative to Group IA suggests that the increased dose of methotrexate may enhance the efficacy of the treatment in reducing swelling in the joints. The comparison group also exhibited a decline in the number of swollen joints, but it was less pronounced, ending at 14.8. Although there is an improvement, the graph highlights that the combination therapy groups, particularly Group IB, are more effective in reducing the swollen joint count compared to monotherapy. 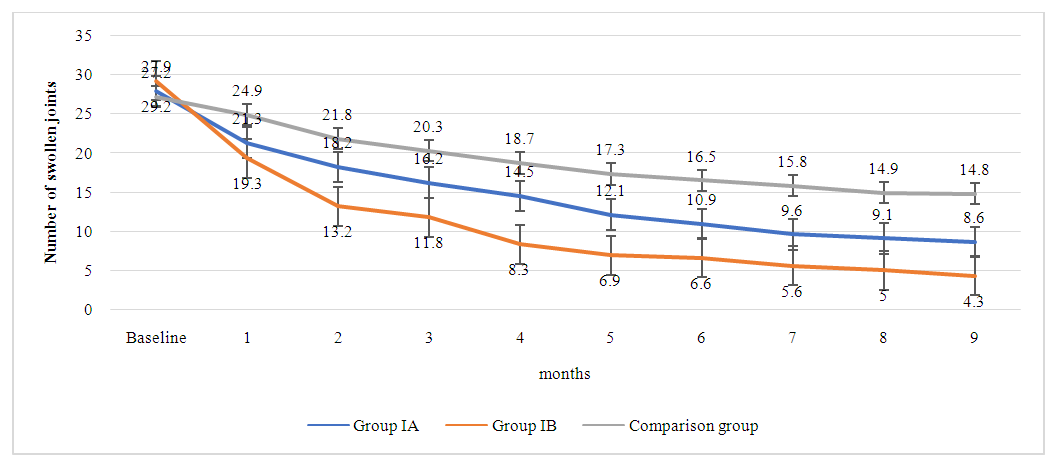 | Picture 4.4. The dynamics of swollen joints count in patients with rheumatoid arthritis across all treatment groups |
The data presented convey the overall effectiveness of the treatments in managing swollen joints, with the steepest decline in Group IB indicating the highest efficacy. This visual representation underscores the benefit of combination therapies, especially with higher methotrexate dosages, in treating the inflammatory symptoms of rheumatoid arthritis.
4. Conclusions
This observational cohort study demonstrated the superior efficacy of combination therapy with adalimumab and methotrexate compared to methotrexate monotherapy in patients with rheumatoid arthritis (RA) who had an inadequate response to methotrexate alone. Over nine months of treatment, patients receiving combination therapy showed significantly higher rates of clinically meaningful improvements in disease activity, as measured by ACR20, ACR50, and ACR70 criteria. Specifically, the combination of adalimumab with a higher dose of methotrexate (15 mg weekly) appeared to provide slightly better outcomes than the combination with a lower dose (7.5 mg weekly), although the difference was not statistically significant.The study also highlighted the importance of considering both the type and dosage of medications in the management of RA to achieve optimal patient outcomes. The consistent reduction in tender and swollen joint counts further supports the effectiveness of combination therapy in controlling disease symptoms and reducing inflammation.In summary, the findings of this study contribute to the growing body of evidence supporting the use of combination therapy with biologic agents and methotrexate in the treatment of RA. They underscore the need for personalized treatment strategies that consider the individual patient's response to therapy and disease severity. Future research should focus on long-term outcomes and the identification of predictors of response to optimize the management of RA and improve the quality of life for patients living with this chronic condition.
References
| [1] | E. Rajaei et al., “Evaluating the relationship between serum level of interleukin-6 and rheumatoid arthritis severity and disease activity,” Current rheumatology reviews, vol. 16, no. 3, pp. 249–255, 2020. |
| [2] | N. d Buchs, F. S. Di Giovine, T. Silvestri, E. Vannier, G. W. Duff, and P. Miossec, “IL-1B and IL-1Ra gene polymorphisms and disease severity in rheumatoid arthritis: interaction with their plasma levels,” Genes & immunity, vol. 2, no. 4, pp. 222–228, 2001. |
| [3] | M. Ziolkowska et al., “High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism,” The Journal of Immunology, vol. 164, no. 5, pp. 2832–2838, 2000. |
| [4] | W. B. Van Den Berg and P. Miossec, “IL-17 as a future therapeutic target for rheumatoid arthritis,” Nature Reviews Rheumatology, vol. 5, no. 10, pp. 549–553, 2009. |
| [5] | S. R. Pickens, M. V. Volin, A. M. Mandelin, J. K. Kolls, R. M. Pope, and S. Shahrara, “IL-17 contributes to angiogenesis in rheumatoid arthritis,” The Journal of Immunology, vol. 184, no. 6, pp. 3233–3241, 2010. |
| [6] | A. Ogata, Y. Kato, S. Higa, and K. Yoshizaki, “IL-6 inhibitor for the treatment of rheumatoid arthritis: a comprehensive review,” Modern rheumatology, vol. 29, no. 2, pp. 258–267, 2019. |
| [7] | M. Narazaki, T. Tanaka, and T. Kishimoto, “The role and therapeutic targeting of IL-6 in rheumatoid arthritis,” Expert Review of Clinical Immunology, vol. 13, no. 6, pp. 535–551, Jun. 2017, doi: 10.1080/1744666X.2017.1295850. |
| [8] | G. W. Kim et al., “IL-6 inhibitors for treatment of rheumatoid arthritis: past, present, and future,” Archives of pharmacal research, vol. 38, pp. 575–584, 2015. |
| [9] | J. S. Smolen and D. Aletaha, “Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges,” Nature Reviews Rheumatology, vol. 11, no. 5, pp. 276–289, 2015. |
| [10] | M. S. Akram et al., “Challenges for biosimilars: focus on rheumatoid arthritis,” Critical Reviews in Biotechnology, vol. 41, no. 1, pp. 121–153, Jan. 2021, doi: 10.1080/07388551.2020.1830746. |
| [11] | G. M. Bartelds et al., “Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis,” Annals of the rheumatic diseases, vol. 66, no. 7, pp. 921–926, 2007. |
| [12] | L. M. Bang and G. M. Keating, “Adalimumab: A Review of its Use in Rheumatoid Arthritis,” BioDrugs, vol. 18, no. 2, pp. 121–139, 2004, doi: 10.2165/00063030-200418020-00005. |
| [13] | A.-F. Radu and S. G. Bungau, “Management of rheumatoid arthritis: an overview,” Cells, vol. 10, no. 11, p. 2857, 2021. |
| [14] | G. S. Firestein, “Evolving concepts of rheumatoid arthritis,” Nature, vol. 423, no. 6937, pp. 356–361, 2003. |
| [15] | K. O. Anderson, L. A. Bradley, L. D. Young, L. K. McDaniel, and C. M. Wise, “Rheumatoid arthritis: review of psychological factors related to etiology, effects, and treatment.,” Psychological bulletin, vol. 98, no. 2, p. 358, 1985. |
| [16] | D. Aletaha and J. S. Smolen, “Diagnosis and management of rheumatoid arthritis: a review,” Jama, vol. 320, no. 13, pp. 1360–1372, 2018. |







 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


