-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(4): 1069-1076
doi:10.5923/j.ajmms.20241404.56
Received: Mar. 19, 2024; Accepted: Apr. 15, 2024; Published: Apr. 19, 2024

Factors of Unfavorable Prognosis in Stage III Colorectal Cancer
Islamov Hurshid Jamshidovich, Tillashayhov Mirzagolib Negmanovich, Egamberdiyev Dilshod Mahmudovich, Kamishov Sergey Viktorivich, Izrailbekova Kamila Shavkatovna, Karahodjaev Botir Shokirovich, Matniyazova Shakar Yakubovna
Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology, Tashkent, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The study and identification of prognostic factors affecting long-term treatment results will allow individualizing the treatment of patients with stage III of the disease. The study obtained the following predictors of the prognosis of disease progression (distant metastases): the degree of tumor invasion (T4), metastatic lesion of regional lymph nodes (N2) and the absence of adjuvant chemotherapy, which showed a significant effect in multivariate analysis.
Keywords: Colorectal cancer, Prognosis, Risk factors, Metastases, Chemotherapy
Cite this paper: Islamov Hurshid Jamshidovich, Tillashayhov Mirzagolib Negmanovich, Egamberdiyev Dilshod Mahmudovich, Kamishov Sergey Viktorivich, Izrailbekova Kamila Shavkatovna, Karahodjaev Botir Shokirovich, Matniyazova Shakar Yakubovna, Factors of Unfavorable Prognosis in Stage III Colorectal Cancer, American Journal of Medicine and Medical Sciences, Vol. 14 No. 4, 2024, pp. 1069-1076. doi: 10.5923/j.ajmms.20241404.56.
1. Introduction
- Colorectal cancer (CRC), due to the increasing incidence, remains a serious health problem worldwide. According to Cancer Today, 1,926,425 cases of the disease and 904,019 deaths from CRC have been registered worldwide. In Asia, more than 960,000 new cases and more than 460,000 deaths were reported in 2022. In 2022, CRC ranks third in the cancer incidence structure (10.3%): among the male population after lung and prostate cancer, among the female population after breast cancer and lung cancer [1].In the Republic of Uzbekistan, according to official statistics, 1,818 patients with colorectal cancer were identified in 2022, of which 290 patients (16.0%) were diagnosed with stage IV disease. 936 patients died from colorectal cancer in the country. The ratio of the deceased to the sick remains high, reflecting the effectiveness of anti–cancer measures, including diagnostic and therapeutic measures, in 2022 - 51.5% [2].Surgery is the main method of treating CRC. For shallow tumors, endoscopic resection is the recommended treatment option, provided the patient is properly monitored [3]. More infiltrative tumors require surgical intervention. The purpose of resection is to remove the tumor and adjacent lymph nodes. In colon cancer, the volume of resection depends on lymphovascular drainage in the tumor area, but should cover a segment of the colon at least 5 cm long on each side of the tumor [4]. It is also important to examine and, if possible, palpate the abdominal and pelvic organs, as well as the abdominal cavity, to ensure that there are no metastases. Partial colectomy can be performed laparoscopically with preservation of the oncological outcome, but with a faster recovery after surgery compared with open surgery [5,6]. Surgical treatment of the disease is possible in patients with stage IV, but the selection of patients for resection of metastases is of great importance. The approach to patients with metastases should be multidisciplinary, while taking into account both technical and prognostic factors [7].Surgical treatment, as an independent method, is effective for colon cancer in stages I-II. In colon cancer of the first stage of the disease, the effectiveness of surgical intervention as an independent treatment method has been proven, in other cases, it is necessary to conduct a preoperative course of radiation therapy [8]. About 25-40% of patients treated with CRC according to the radical program (stages I-II) develop a relapse of the disease or distant metastases appear. Distant metastases, among patients with stage III of the disease, appear in almost 35% of cases after potentially radical treatment. The progression of the disease, in this case, is due to the activation of tumor cells that migrated even before surgery from the primary localization of the tumor. Adjuvant chemotherapy is prescribed worldwide to solve this problem [9,10].Thus, the increase in the incidence of CRC worldwide, including in the Republic of Uzbekistan, the high frequency of disease progression after treatment determines the relevance of this issue for the oncological service. The study and identification of prognostic factors affecting long-term treatment results will allow individualizing the treatment of patients with stage III of the disease.
2. Material and Methods
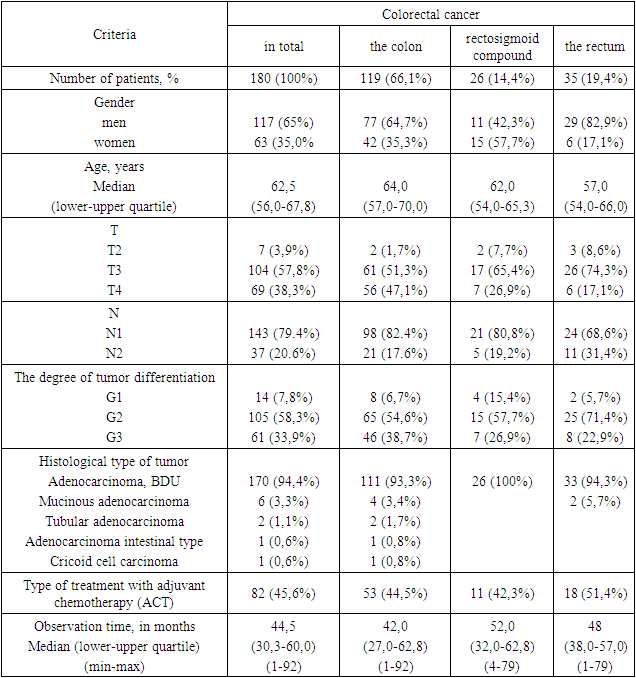
- The object of the study was information about 180 patients treated in the oncological coloproctology department of the Republican Specialized Scientific and Practical Center of Oncology and Medical Radiology in the period from 01.01.2016 to 12/31/2020. The study is retrospective. The criteria for inclusion in the study are primary colorectal cancer, stage III of the disease (N1–N2), a mandatory surgical component of treatment, and the absence of serious concomitant pathology.To designate a tumor, depending on the localization, the code of the International Classification of Diseases of the 10th revision (ICD-10) was used, according to which C18 is colon cancer, C19 is rectosigmoid compound cancer, C20 is rectal cancer. The international classification of TNM was used to stage the disease. The main characteristics of the patients included in the study are presented in Table 1.
|
3. Results
- The five-year overall survival in the study group was 66.4±4.3%, the median survival was not achieved. Figure 1 shows the overall survival rate of patients depending on the localization of the tumor process. Thus, the 5-year of general detectability (GD) in colon cancer was 74.2±4.9%, in rectosigmoid compound cancer – 55.1±10.2%, in rectal cancer – 55.6±10.0%. The median GD was achieved for rectosigmoid junction cancer – 62 months and rectal cancer – 63 months.
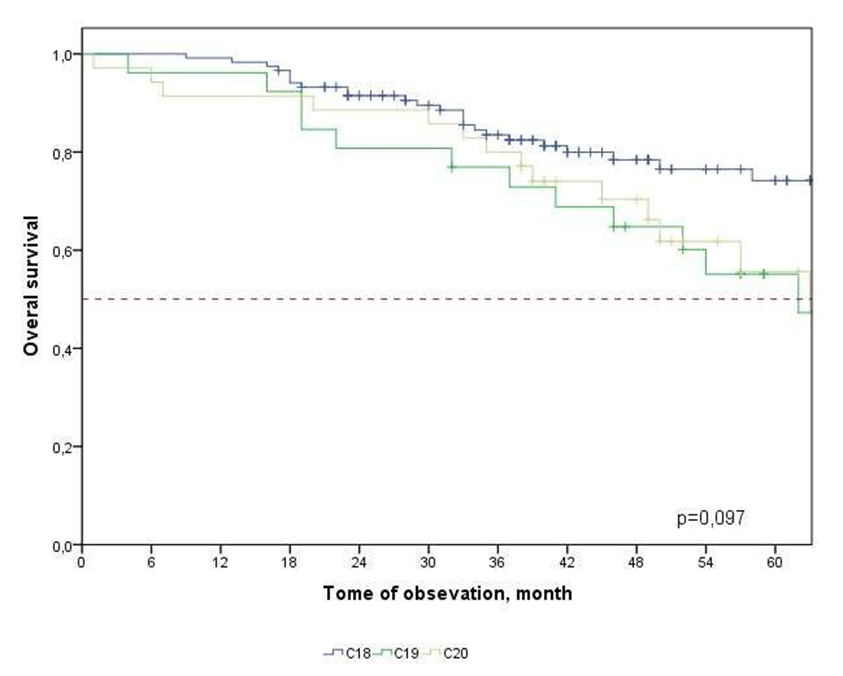 | Figure 1. Overall survival of patients with CRC depending on the localization of the tumor process |
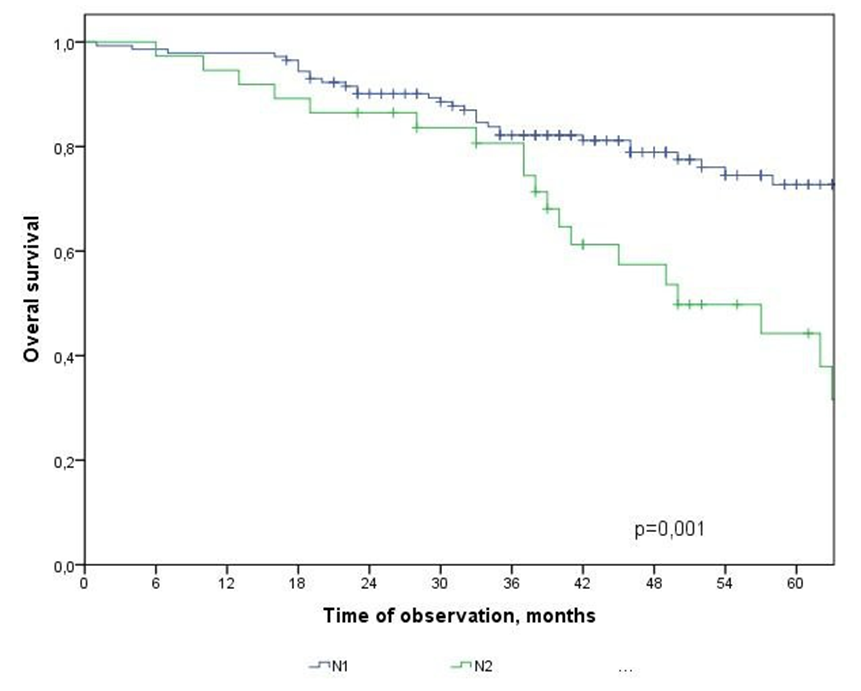 | Figure 2. Overall survival of CRC patients depending on the degree of metastatic lesion of regional lymph nodes |
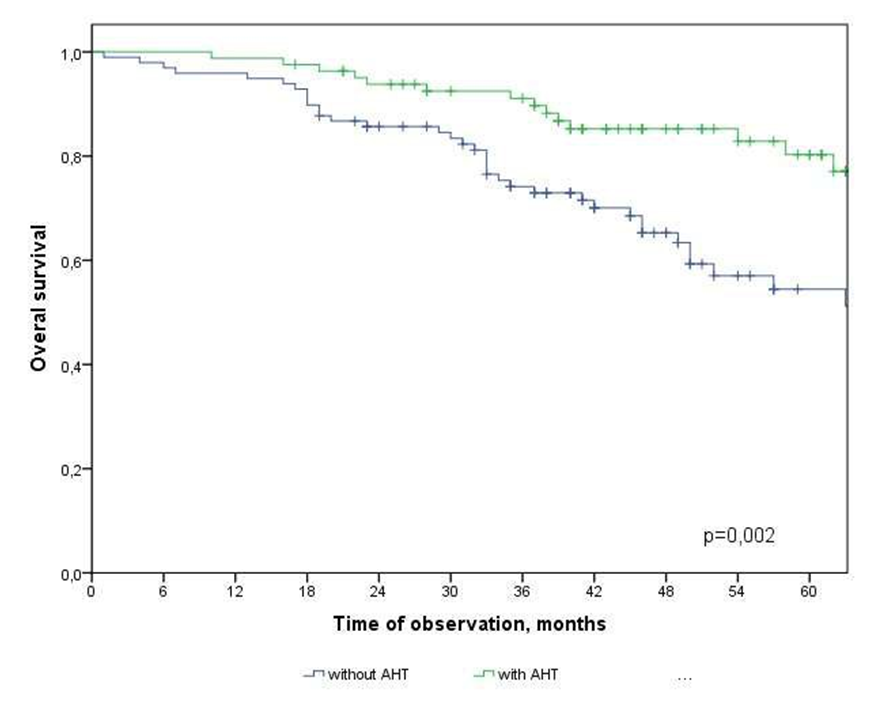 | Figure 3. Overall survival of CRC patients depending on the type of treatment |
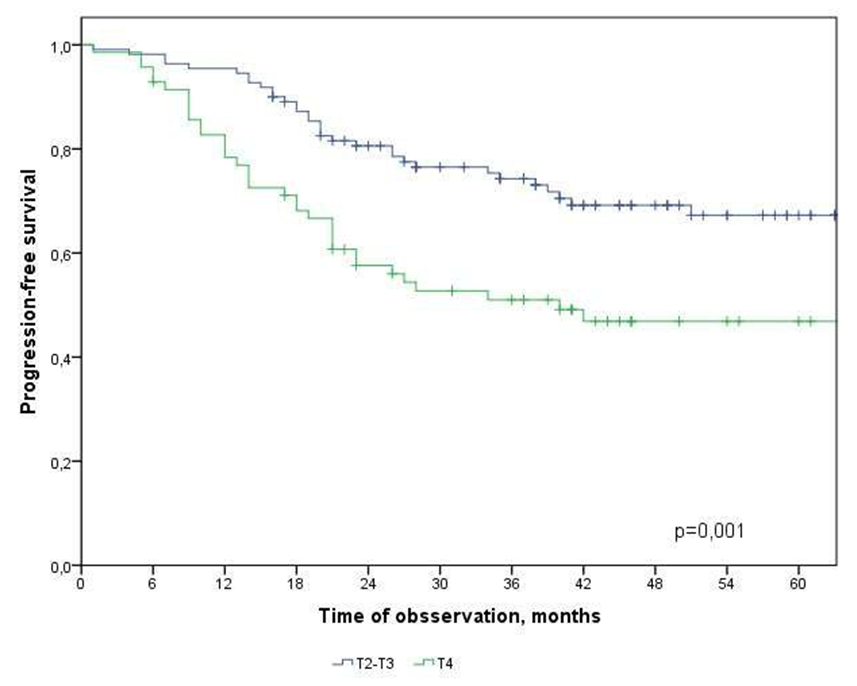 | Figure 4. Progression-free survival in CRC patients depending on the depth of invasion |
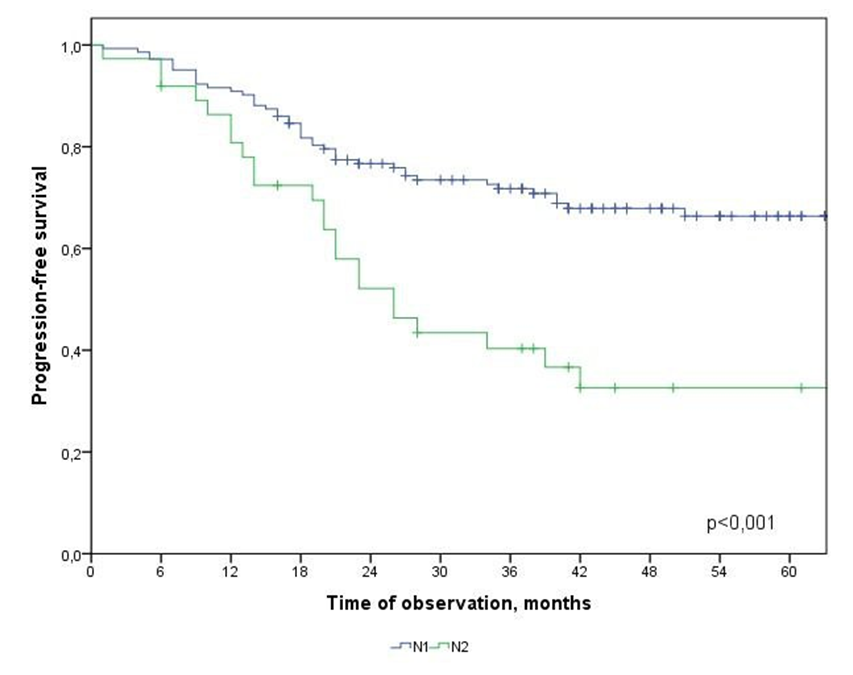 | Figure 5. Progression-free survival in CRC patients depending on metastatic lesion of regional lymph nodes |
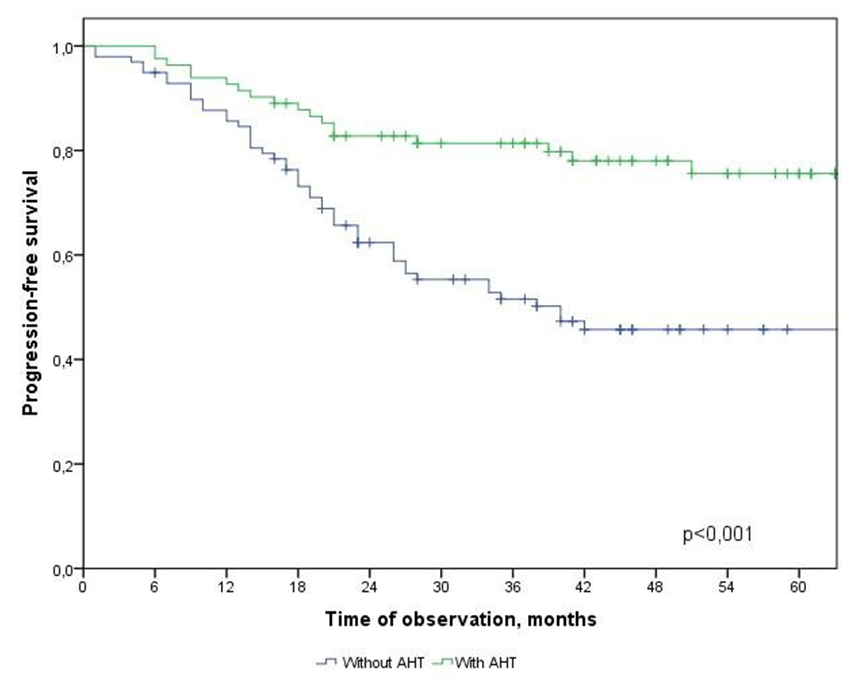 | Figure 6. Progression-free survival in CRC patients depending on the type of treatment |
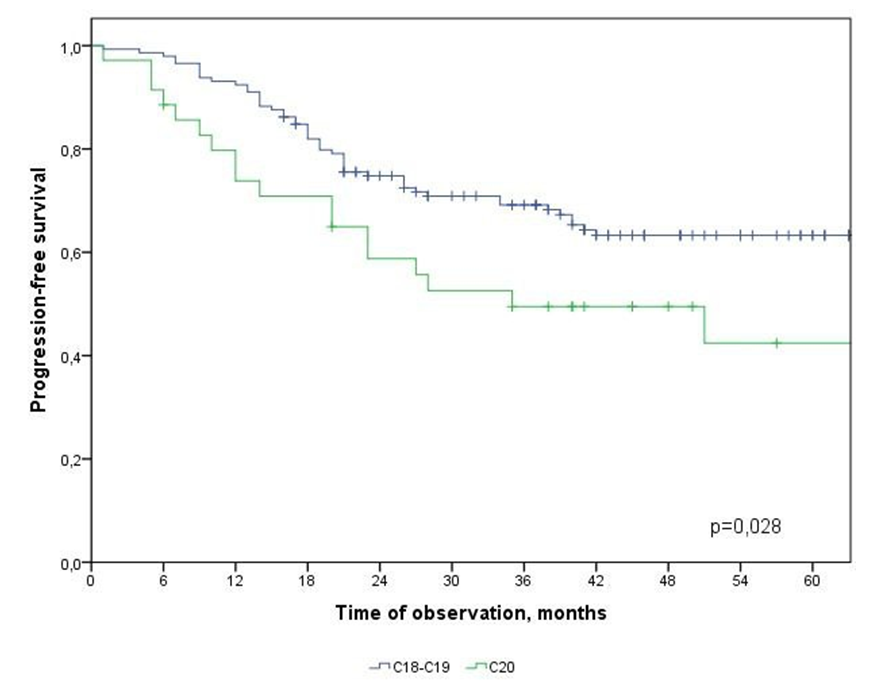 | Figure 7. Progression-free survival in CRC patients depending on the primary location of the tumor |
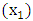 Т2-Т3 and Т4
Т2-Т3 and Т4 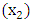 N: N1 and N2
N: N1 and N2 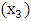 АCТ: - with АCТ и without АCТ
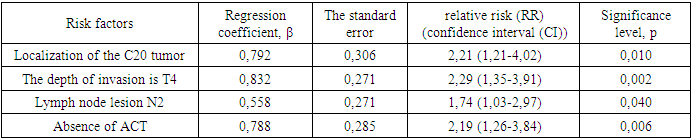
АCТ: - with АCТ и without АCТ 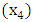 The results of the multivariate analysis are presented in Table 2, the statistical significance of the model was p<0.001.
The results of the multivariate analysis are presented in Table 2, the statistical significance of the model was p<0.001.
|
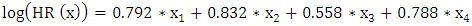
4. Conclusions
- In the study, the overall survival of CRC patients in the group of patients with N1 was statistically (p=0.001) significantly higher (74.5±4.3%) than in the group of patients with N2 (44.2±9.7%); among patients who did not receive ACT, survival was statistically significantly lower (p=0.002) than in the group receiving ACT.The study obtained the following predictors of the prognosis of disease progression (distant metastases): the degree of tumor invasion (T4), metastatic lesion of regional lymph nodes (N2) and the absence of adjuvant chemotherapy, which showed a significant effect in multivariate analysis. Thus, the risk of CRC progression increases with: tumor localization C20 (RR=2.21, 95% CI 1.21-4.02, p=0.010), depth of invasion into the intestinal wall T4 (RR=2.29; 95% CI 1.35-3.91, p=0.002), metastic lesion of regional lymph nodes N2 (RR=1.74, 95% CI 1.03-2.97, p=0.040), absence of ACT (RR=2.19; 95% DI 1.26-3.84, p=0.006).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML