-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(4): 1003-1006
doi:10.5923/j.ajmms.20241404.41
Received: Mar. 12, 2024; Accepted: Apr. 10, 2024; Published: Apr. 18, 2024

Viral Diarrhea in HIV-Infected Children on the Background of Immunological Changes
Otajanov Shamsiddin Zarifbayevich, Gulnara Karimovna Khudaykulova, Makhbuba Teshaevna Muminova
Tashkent Medical Academy, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this article, the clinical and laboratory features and changes of viral diarrhea in HIV-infected children were studied and analyzed.
Keywords: HIV infection, Diarrhea, Rotavirus, Adenovirus, Norovirus
Cite this paper: Otajanov Shamsiddin Zarifbayevich, Gulnara Karimovna Khudaykulova, Makhbuba Teshaevna Muminova, Viral Diarrhea in HIV-Infected Children on the Background of Immunological Changes, American Journal of Medicine and Medical Sciences, Vol. 14 No. 4, 2024, pp. 1003-1006. doi: 10.5923/j.ajmms.20241404.41.
1. Relevance
- The spread of HIV infection in the world is one of the biggest threats to the security of humanity today. This problem was recognized by the UN General Assembly in its founding document "Declaration of commitment to fight against HIV/AIDS". According to the WHO Joint Program on Human Immunodeficiency Virus (HIV) and Acquired Immune Deficiency Syndrome (AIDS), by 2021, 38.4 million people will be living with HIV, 1.7 million and they are children. Babies get HIV from their mothers at three different times: during fetal life (when the baby is in the womb), during delivery, and during breastfeeding [1,5,7].The problem of acute intestinal infections remains relevant even today, because it is characterized by a wide spread, severe, complicated forms of the disease, and a significant frequency of the development of digestive diseases after an infectious disease. Viral infections of the gastrointestinal system are less known to general practitioners than bacteria: viruses account for 30-40% of acute episodes of diarrhea in young children, among which rotavirus infection plays the first role (60-80%). Viral damage to the gastrointestinal tract in immunocompromised patients: recipients of bone marrow and other organs, patients undergoing chemotherapy, HIV and AIDS is an equally serious problem. In this group, even with proper treatment, death from severe forms of viral infection is alarmingly high. In recent years, there have been publications about the spread of viral diarrhea not of rotavirus etiology. This made it necessary to summarize new information on the most common etiological factors that cause acute gastroenteritis epidemics. Features of viral diarrhea are the acute onset of the disease with rapidly developing ecchymosis; positive dynamics with correct and quickly organized rehydration therapy, high resistance to the external environment and high infectivity despite the measures taken against the epidemic, asymptomatic carriage and continued release of the virus into the external environment after clinical recovery, as well as the still discussed infection spread by aerosol. The pathogenetic feature of the rapid manifestation of dehydration is the breakdown of disaccharides and lipids without changing the levels of sAMF and adenyl cyclase, which leads to damage to enterocytes and the appearance of secondary lactase deficiency in the future [5,8,11].Many pathogens can cause enteritis or enterocolitis in HIV-infected patients and cause acute, chronic, or recurrent diarrhea. Salmonella spp., Shigella spp., Campylobacter jejuni, G.lamblica, Cryptosporidium parvum, Cytomegalovirus, Adenovirus, Rotavirus, Herpes simplex are the most common causes of intestinal infections in children. According to researches, up to 80% of viruses play the main role in the etiology of intestinal infections in children. The most studied rotavirus causes diarrhea in up to 50%, noravirus up to 30% in developed countries. The list of viruses that cause intestinal infections is constantly growing, when diagnosing these pathogens, astroviruses, adenoviruses, and toraviruses are identified [4,7,14].To assess the safety of antiretroviral therapy, transaminases, pancreatic enzyme activity, and bilirubin should be monitored continuously. If acute hepatitis, acute pancreatitis is observed under the influence of antiretroviral drugs, it is necessary to change the drug. Thus, GIS damage in HIV-infected children plays an important role in the clinical picture of the disease and determines the course and outcome of the disease. Children infected with HIV may develop diarrhea syndrome due to pathological changes in the intestines in various fecal-oral infectious diseases. In the clinic of HIV-infection, the impact of GIS dysfunction on nutrition and immune status takes a special place, these changes lead to children's growth and physical formation, CNS development lags behind. In HIV-infection, all parts of GIS are affected [6,7].
2. The Purpose of the Study
- To study of clinical and laboratory features of viral diarrhea in HIV-infected children.
3. Material and Methods
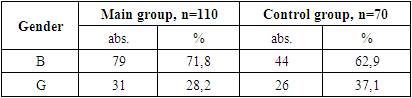
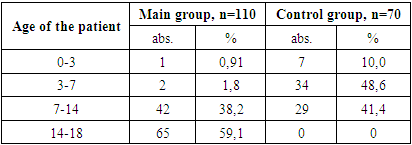
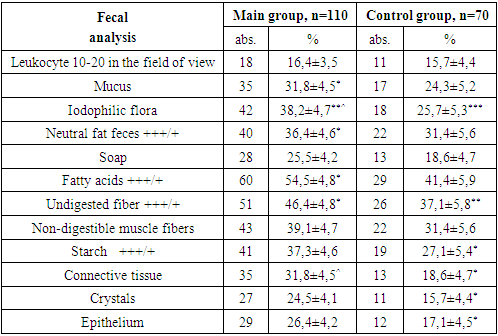
- During the study, children under 18 years of age were divided into two groups: the main group consisted of 110 HIV-infected children with viral diarrhea, the control group consisted of 70 children without HIV infection with only viral diarrhea.The diagnosis of HIV infection in children was made on the basis of the “National Clinical Report on the organization and implementation of medical care for persons with confirmed HIV status” No. 206 dated 08/19/2021 of the Ministry of Health of the Republic of Uzbekistan and No. 122 dated 03/25/2015 “On improvement measures to combat typhoid fever, paratyphoid fever, salmonellosis and acute intestinal diseases.”When assessing the severity of acute infectious diarrhea in HIV-infected children, an assessment is made of the degree of dehydration (dehydration according to WHO) that developed as a result of diarrhea in sick children, the daily amount and duration of diarrhea, as well as the shape, consistency, smell, color, amount of stool, existing pathological impurities. The diagnosis was established on the basis of patient complaints, clinical, anthropometric, serological, bacteriological, immunological, virological and instrumental studies.
|
|
|
|
|
|
|
4. Conclusions
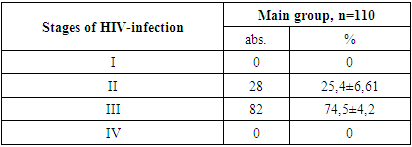
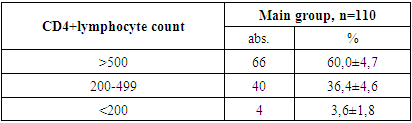
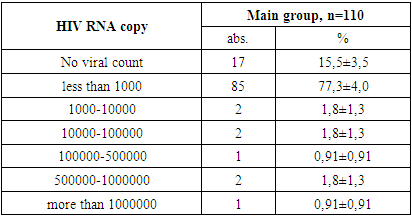
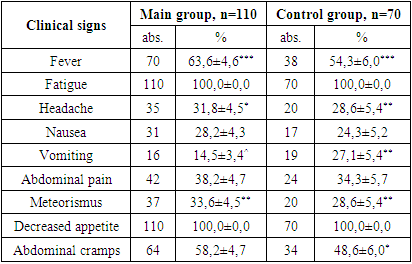
- 1. In the patients in the main and control groups, the disease occurred mainly in boys (71.8% and 62.9% of cases).2. The second and third clinical stages of HIV infection were established in the main group of patients (in 25.4% and 74.5% of cases).3. When studying the immunological features in the patients of the main group, severe immunodeficiency was almost not found (3.6% of cases), the viral load was found to be mostly less than 1000 (77.3% of cases).4. When examining the clinical signs of patients, weakness and loss of appetite were found in all cases (100%) in all the main and control groups. Among the symptoms of intoxication, fever was observed more often in the main and control groups (63.6% and 54.3% of cases, respectively), while headache occurred in almost ⅓ of patients in the main and control groups.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML