-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(4): 968-973
doi:10.5923/j.ajmms.20241404.35
Received: Mar. 22, 2024; Accepted: Apr. 7, 2024; Published: Apr. 11, 2024

Assessment of Central Hemodynamics and External Respiration in Patients after COVID-19 Related Pneumonia
Nazarov F. Y., Yarmuxamedova S. Kh.
Chair of Internal Medicine and propaedeutics, Samarkand State Medical University, Samarkand, Uzbekistan
Correspondence to: Nazarov F. Y., Chair of Internal Medicine and propaedeutics, Samarkand State Medical University, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

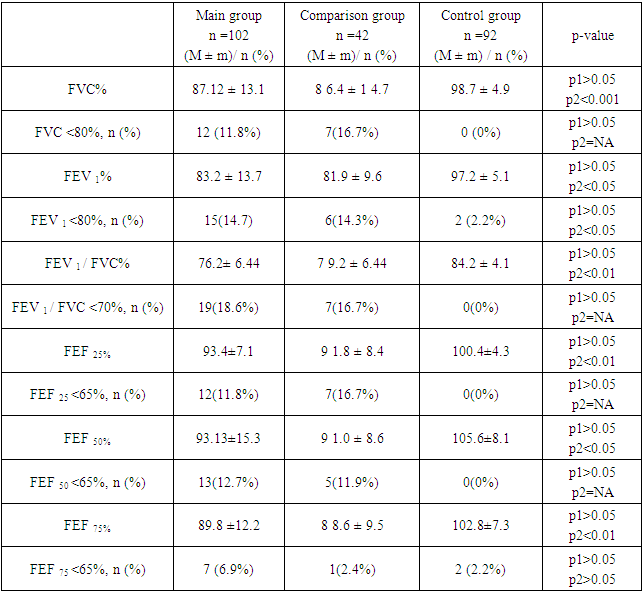
The COVID-19 pandemic, caused by the novel coronavirus SARS-CoV-2, has significantly impacted global health, leading to a wide range of clinical manifestations. This study aims to evaluate the indicators of central hemodynamics and external respiration in patients who have recovered from community-acquired coronavirus pneumonia (CCP). An observational study was conducted at the Cardiology Department of the Samarkand State Medical University, Uzbekistan, from 2020 to 2021. A total of 102 patients aged between 40 and 55 years, who were diagnosed with CCP and had undergone treatment and examination at the Cardiology Department, were included in the study. The study utilized clinical data, laboratory investigations, and instrumental methods such as spirometry and transthoracic echocardiography (TTE) to assess central hemodynamics and external respiration. The results revealed significant clinical and laboratory alterations in patients recovering from CCP. Elevated levels of leukocytes, neutrophils, and markers of inflammation and cardiac injury such as C-reactive protein and troponin-I were observed. Echocardiographic evaluations indicated moderate impairments in heart function among CCP survivors, with a substantial proportion of patients exhibiting left ventricular hypertrophy and dysfunction. Spirometry results demonstrated reduced forced vital capacity (FVC) and forced expiratory volume in one second (FEV1), indicating persistent respiratory impairment. The study underscores the importance of comprehensive post-recovery care for patients who have survived CCP, highlighting the need for ongoing monitoring and tailored management strategies to address the long-term health consequences of the disease.
Keywords: COVID-19, Community-Acquired Coronavirus Pneumonia, Central Hemodynamics, External Respiration, Post-COVID Syndrome
Cite this paper: Nazarov F. Y., Yarmuxamedova S. Kh., Assessment of Central Hemodynamics and External Respiration in Patients after COVID-19 Related Pneumonia, American Journal of Medicine and Medical Sciences, Vol. 14 No. 4, 2024, pp. 968-973. doi: 10.5923/j.ajmms.20241404.35.
1. Introduction
- The COVID-19 pandemic, caused by the novel coronavirus SARS-CoV-2, has had a profound impact on global health, leading to a wide range of clinical manifestations, from asymptomatic cases to severe respiratory distress syndrome and multi-organ failure. One of the critical areas affected by the virus is the cardiovascular and respiratory systems. The interplay between the virus and these systems is complex, with emerging evidence suggesting that COVID-19 can lead to significant alterations in central hemodynamics and external respiration, even in patients who have recovered from the acute phase of the infection [1,5,9].Central hemodynamics, which encompasses the functioning of the heart and major blood vessels, is crucial for maintaining adequate tissue perfusion and oxygenation. Disruptions in this system can lead to a cascade of physiological imbalances, affecting the body's ability to deliver oxygen to vital organs. External respiration, the process of gas exchange between the external environment and the body's circulatory system, is equally vital. Impairments in this process can compromise oxygen uptake and carbon dioxide elimination, leading to hypoxemia and hypercapnia [2,4,12,16-18].The long-term consequences of COVID-19 on central hemodynamics and external respiration are still being unraveled. There is growing concern that patients who have recovered from the acute phase of the infection may continue to experience cardiovascular and respiratory complications, a condition often referred to as "post-COVID-19 syndrome" or "long COVID." These complications can manifest as persistent symptoms such as dyspnea, chest pain, and fatigue, which can significantly impact the quality of life and increase the risk of long-term morbidity [3-7,12,19].COVID-19 primarily affects the respiratory system, targeting the lungs and airways. The virus enters host cells via the angiotensin-converting enzyme 2 (ACE2) receptor, which is highly expressed in the respiratory tract. This viral entry can lead to a range of respiratory complications, including pneumonia, acute respiratory distress syndrome (ARDS), and respiratory failure [10-14].The pathophysiology of COVID-19 respiratory involvement involves a cascade of events, starting with viral replication in respiratory epithelial cells, leading to an inflammatory response characterized by cytokine release (cytokine storm), immune cell infiltration, and tissue damage. This inflammatory cascade can result in alveolar damage, impaired gas exchange, and respiratory dysfunction [4-9].Clinical manifestations of COVID-19 respiratory involvement vary widely, from mild symptoms such as cough and shortness of breath to severe respiratory distress requiring mechanical ventilation. The severity of respiratory complications often correlates with patient age, comorbidities, and immune response [13-17].A critical aspect of COVID-19 pathology is the cytokine response triggered by the viral infection. Cytokines are small proteins released by cells, particularly immune cells, that play a crucial role in cell signaling and the regulation of immune responses. In the context of COVID-19, the virus-induced activation of the immune system can lead to an excessive release of cytokines, a phenomenon known as a "cytokine storm." [12,17,19]The cytokine storm is characterized by elevated levels of various cytokines, including interleukin-6 (IL-6), interleukin-8 (IL-8), tumor necrosis factor-alpha (TNF-α), and others. This hyperactive immune response can contribute to the severity of the disease by causing widespread inflammation, tissue damage, and multi-organ dysfunction [12,15,17].In this context, our study aims to evaluate the indicators of central hemodynamics and external respiration in patients who have recovered from community-acquired coronavirus pneumonia. By examining these parameters, we seek to understand the extent of residual cardiovascular and respiratory dysfunction in this population and to identify potential risk factors associated with adverse outcomes. Such insights are crucial for guiding the clinical management of post-COVID-19 patients and for developing targeted rehabilitation strategies to mitigate the long-term effects of the disease.
2. Materials and Methods
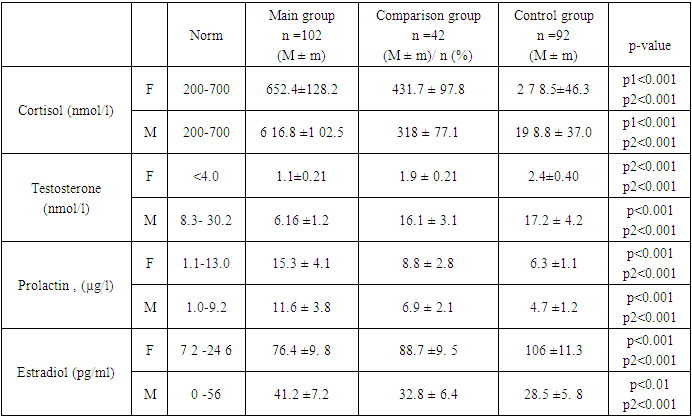
- This observational study was conducted at the Cardiology Department of the Samarkand State Medical University, Uzbekistan, from 2020 to 2021. The study aimed to evaluate the central hemodynamics and external respiration parameters in patients who had recovered from community-acquired coronavirus pneumonia (CCP).A total of 102 patients aged between 40 and 55 years, who were diagnosed with CCP and had undergone treatment and examination at the Cardiology Department, were included in the study. The inclusion criteria were as follows: age between 40 and 55 years, diagnosis of community-acquired coronavirus pneumonia. Patients with a history of chronic cardiovascular or respiratory diseases were excluded from the study.Clinical data, including demographic information, medical history, and physical examination findings, were collected using standardized forms. Laboratory investigations comprised a complete blood count, biochemical analysis, and assessment of hormone levels (testosterone, cortisol, estradiol, prolactin) and pro-inflammatory cytokines (IL-6 and IL-8) in serum. Instrumental methods included spirometry for evaluating external respiration and transthoracic echocardiography (TTE) along with Doppler imaging for assessing central hemodynamics.Central hemodynamics were evaluated using TTE and Doppler imaging, focusing on parameters such as cardiac output, ejection fraction, and blood flow velocities. External respiration was assessed through spirometry, measuring parameters such as forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and the FEV1/FVC ratio.Data were analyzed using descriptive statistics, including means and standard deviations for continuous variables, and frequencies and percentages for categorical variables. Comparative analyses were performed using t-tests for continuous variables and chi-square tests for categorical variables. A p-value of less than 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS software (version 26.0).The study protocol was approved by the Institutional Review Board of the Samarkand State Medical University. All participants provided informed consent before enrollment in the study. The study was conducted in accordance with the Declaration of Helsinki and ethical guidelines for human research.To ensure the accuracy and reliability of the data, all measurements were performed by trained and experienced medical personnel. The equipment used for clinical and laboratory measurements was regularly calibrated and maintained according to the manufacturer's guidelines.
3. Results
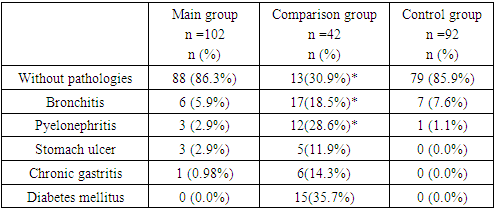
- The analysis of the anamnestic data revealed that 88 (86.3%) patients with community-acquired pneumonia (CCP) had no preceding pathologies. Chronic bronchitis was the FEF common pathology among the patients in the main group, reported in the history of 5.9% of the patients, with no signs of recurrence in the last 2 years. Additionally, three patients (2.9%) had chronic pyelonephritis prior to hospitalization for CCP, also without significant signs of the disease in the last 2 years.
|
|
|
4. Conclusions
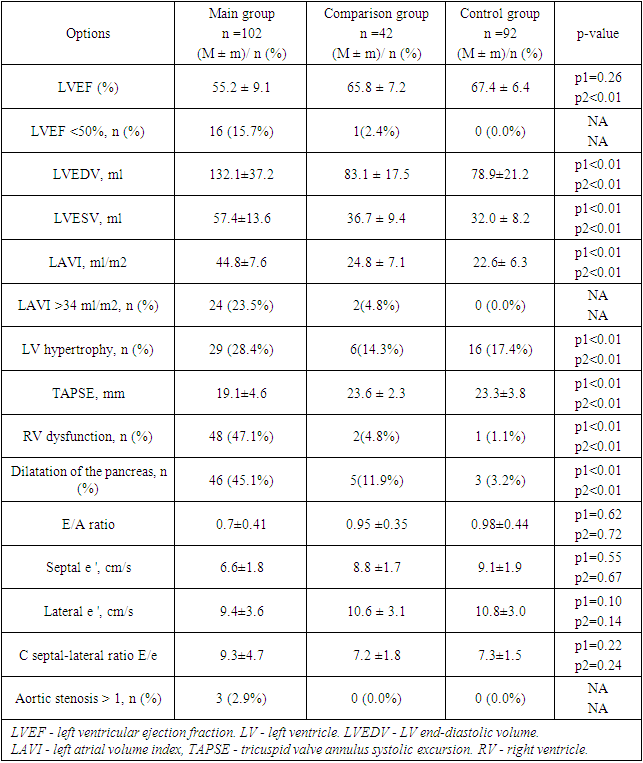
- Our study reveals significant clinical and laboratory alterations in patients recovering from community-acquired coronavirus pneumonia (CCP). The majority of these patients had no preceding pathologies, with chronic bronchitis being the most commonly reported condition. Laboratory findings showed elevated levels of leukocytes, neutrophils, and markers of inflammation and cardiac injury, such as C-reactive protein and troponin-I. Echocardiographic evaluations indicate moderate impairments in heart function among CCP survivors, with a substantial proportion of patients exhibiting left ventricular hypertrophy and dysfunction. This underscores the potential long-term cardiovascular effects of CCP.
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML