-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(4): 934-939
doi:10.5923/j.ajmms.20241404.28
Received: Mar. 6, 2024; Accepted: Apr. 7, 2024; Published: Apr. 11, 2024

Effects of Sodium-Glucose Cotransporter Type 2 Inhibitors on Fibrosis Processes in Chronic Heart Failure Developed on the Basis of Rheumatic Heart Defects
Gadaev Abdigaffar Gadaevich1, Qayumov Laziz Kholmurodovich2
1Tashkent Medical Academy, Tashkent, Uzbekistan
2Bukhara State Medical Institute named after Abu Ali ibn Sina, Bukhara, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In chronic heart failure developed on the basis of rheumatic heart defects, inflammatory processes are long and hidden. This ultimately leads to the development of fibrotic processes in the interstitial tissue of the heart. In the article, high levels of galectin-3 were found in the blood of patients with chronic heart failure developed on the basis of rheumatic heart defects. The regression analysis showed that the left ventricular ejection fraction decreased in parallel with the increase in galectin-3 index before treatment. Reduction of galectin-3 in the blood and positive changes in the left ventricular ejection fraction after standard treatment with sodium glucose cotransporter type 2 inhibitors in patients.
Keywords: Chronic heart failure, Ischemic heart disease, Angiotensin-converting enzyme inhibitors, Galectin-3
Cite this paper: Gadaev Abdigaffar Gadaevich, Qayumov Laziz Kholmurodovich, Effects of Sodium-Glucose Cotransporter Type 2 Inhibitors on Fibrosis Processes in Chronic Heart Failure Developed on the Basis of Rheumatic Heart Defects, American Journal of Medicine and Medical Sciences, Vol. 14 No. 4, 2024, pp. 934-939. doi: 10.5923/j.ajmms.20241404.28.
1. Introduction
- Chronic diseases, including chronic heart failure (CHF), are one of the leading causes of disability and death in the population. Currently, 64.3 million people in the world suffer from CHF, and about 50% of them die within 5 years [7,8].In observations of the world's population, CHF is recorded in 0.3% to 5.3% of the population. In the United States of America (USA), these figures are 2.4-2.6% and among those over 60 years of age, it is 10%. In this country, from 5.8% in 1999, these numbers reached 9.9% in 2019. With increasing age, the incidence of this serious complication is increasing among the elderly, together with arterial hypertension (AH) and ischemic heart disease (IHD), which are its main causes [18].It is known that IHD and AH are the leading causes of CHF. At the same time, in the analyzes presented in the literature, it was noted that rheumatic heart defects are of great importance among the non-ischemic causes of the development of CHF. These sources show that rheumatic disease is more common among young children and elderly people. The disease is associated with immune changes against group A streptococci, and primarily mitral and aortic valves are affected [16]. In women, rheumatism is associated with more mitral valves and its prolapse, and in men, regurgitation or stenosis of aortic valves is noted [14,15].According to various sources, the occurrence of CHF in this group of patients ranges from 4% to 14%. In particular, in an observation conducted by J. McMurray and co-authors, a well-known researcher dealing with the problems of CHF in Scotland, 8% of patients had CHF developed on the basis of rheumatic heart defects [15].Activation of the immune system and systemic inflammatory processes play an important role in the progression and development of the disease in patients with rheumatic heart defects. In CHF, regardless of the etiology of the disease, the amount of pro-inflammatory cytokines in the blood serum was found to be significantly higher than the normal values. Hypoxic processes primarily stimulate the production of hypoxia-inducible factor 1 and α-tumor necrosis factor (α-TNF) in cardiomyocytes, leading to the activation of monocytes and macrophages [6]. Their activation increases the synthesis of a number of inflammatory cytokines and causes deeper damage to the myocardial cells that have undergone ischemia [4].In a number of scientific studies, it has been proven that galectin-3 in the blood serum of patients with CHF causes myocardial hypertrophy and has a stimulating effect on fibrosis processes, which are considered important in heart remodeling, and shows the progression of the disease and the development of unpleasant complications [5].The use of alectin-3 as a biomarker of fibrotic changes in the myocardium and heart failure is increasingly attracting the attention of leading researchers in the world. Galectin-3 belongs to the β-galactoside binding protein family and is produced by many cells. Neutrophils, macrophages, fibroblasts and osteoclasts belong to this group of cells [11]. It has chemotoxic properties against macrophages and monocytes, enhances pro-inflammatory signals, increases neutrophil adhesion and release of pro-inflammatory factors from leukocytes, participates in phagocytosis of neutrophils by macrophages [10].However, the above-mentioned observations were made in advanced cases based on CHF arterial hypertension and IHD. Blood levels of galectin-3 have not been studied in patients with this severe complication resulting from rheumatic heart disease. It is known that the processes observed in the body in rheumatism are somewhat different from arterial hypertension and IHD. From this point of view, the study of galectin-3 in the blood of patients with CHF developed on the basis of rheumatic defects is not only of scientific, but also of practical importance.Currently, angiotensin-converting enzyme inhibitors (AAFIs) or angiotensin receptor antagonists with neprilysin inhibitors (ARNI), β-adrenoblockers (BAB) and mineralocorticoid receptor antagonists (MKRA) are widely used in the treatment of CHF [2,3,12,17].By the European Society of Cardiology, MKRA in combination with AAFI or ARA and β-AB is included in 1 class and A proven group level (1 A) in the treatment of CHF [17].But most of the observations were made on the basis of IHD and AH in developed SUE or in patients with type II diabetes. At the same time, the effects of this group of drugs on heart defects developed as a result of rheumatism and CHF caused by it have been studied very little, and some contradictory opinions have been expressed in them [1,13].Unfortunately, until now, clear evidence-based principles have not been created for the drug treatment of CHF developed on the basis of chronic rheumatic heart defects. Today, there are few recommendations based on expert consensus. Of course, this is related to the complex hemodynamic changes observed in different types of wigs. In recent years, as in a number of developed countries, surgical treatment of heart defects has been systematically introduced in our republic. But despite this, CHF developed due to rheumatic heart defects is still common among the population. From this point of view, the application of new drug groups in their treatment is of practical importance. Taking into account the above, we have recommended empagliflozin, a sodium-glucose cotransporter type 2 inhibitor, as part of the general standard for the treatment of CHF, and we have set ourselves the goal of evaluating its effectiveness in the treatment of CHF developed on the basis of rheumatic defects.
2. Materials and Styles
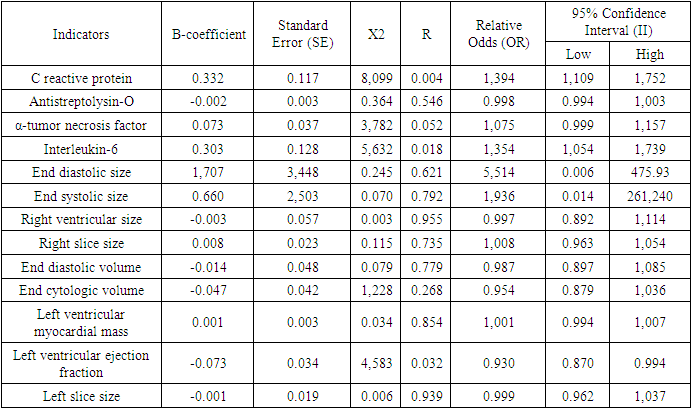
- This planned dissertation work was carried out in 2022 and 2023 in the patients with rheumatic heart failure and developed CHF on the basis of IHD, who were treated at the regional branch of the Bukhara regional branch of the specialized cardiology scientific and practical medical center of the Republic of Uzbekistan and at the multidisciplinary medical center of the Bukhara region. Based on the assigned tasks, the scientific work was carried out as follows. 120 patients with CHF developed on the basis of rheumatic heart disease were included in our observation as the main group. Their average age was 46.8±1.3, men were 41 (34.2%) and women were 79 (65.8%). The average duration of chronic rheumatic heart disease was 12.84±0.45 years. The main group consisted of 48 (40%) patients who underwent surgery and 72 (60%) patients who did not.40 patients with developed CHF based on IHD were included in the control group. Their average age was 64.8±2.3, men were 26 (65%) and women were 14 (35%). The period of illness of the patients with IHD was on average 8.5±0.6 years.In all patients and in the comparison group, the laboratory [general blood analysis, general urinalysis, alanine aminotransferase, aspartate aminotransferase, bilirubin, urea, creatinine, C-reactive protein, antistreptolysin] and instrumental [electrocardiography, echocardiography, ultrasound examination, lung X-ray] tests were performed. interleukin-6, α-tumor necrosis factor, galectin-3 were detected in blood serum.Patients in the main and control groups were prescribed the standard treatment of CHF (angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists, β-blockers, mineralocorticoid receptor antagonists and diuretics, cardiac glycosides, antiarrhythmic drugs according to the indications). In this case, an individual approach was taken based on the general condition of each patient in the selection of treatments. However, empagliflozin, a type 2 inhibitor of glucose sodium cotransporter, was recommended to all patients on the basis of the above-mentioned standard treatment complex.Quantitative index of galectin-3 in blood serum was determined by enzyme immunoassay using human Galectin-3 ELISA (Germany) reagents. A standard with a molecular weight of 26 kDa was used in the set of reagents used for its determination. Test sensitivity was 0.29 ng/ml. Galectin-3 reference indicator was equal to 8.6 [3.7;11.7] ng/ml.Research results and discussion. In recent years, a number of studies have shown that fibrotic processes in the heart muscle play an important role in the occurrence and stable course of CHF. It is known that fibrotic processes in the heart muscle are one of the leading causes of its remodeling. Therefore, the study of fibrotic processes and their markers in CHF is important in predicting the course of the disease and choosing a monad treatment plan. In a number of scientific studies, it has been proven that galectin-3 in the blood serum of patients with SUE causes myocardial hypertrophy and has a stimulating effect on fibrosis processes, which are considered important in heart remodeling, and shows the progression of the disease and the development of unpleasant complications [9]. In most of the studies, the development of fibrotic processes in CHF developed mainly on the basis of YuIK was studied in detail. However, fibrosis processes, especially the amount of galectin-3 and its correlation with a number of indicators, have not been studied in SUE developed on the basis of rheumatic defects. Taking into account the above, we studied galectin-3 in the blood serum of the patients included in our study, its relationship with other indicators, and the effect of empagliflozin, an inhibitor of sodium glucose cotransporter type 2, on them.Galectin 3 index was 18.92±0.6 and 18.2±0.8 ng/ml before treatment in the main and control groups, respectively. When they were compared, no reliable difference (p>0.05) was found. Galectin-3 decreased reliably (p<0.01) from 18.92±0.6 ng/ml to 16.7±0.4 ng in the group with primary, i.e. rheumatic, developed CHF after standard treatment with empagliflozin-containing CHF. In the control group, the amount of galectin-3 was 18.2±0.8 ng/ml before treatment and 13.6±0.9 ng/ml after treatment, and a highly reliable (p<0.001) difference was noted.The obtained results confirmed that fibrotic processes are more evident in CHF developed on the basis of rheumatic heart defects, and although the studied drug had a positive effect (p<0.01), the indicator was lower than the control group (p<0.001). However, it should be noted that empagliflozin has a positive effect on fibrosis processes developed on the basis of rheumatic heart defects, which is related to its reduction of galectin-3 in the blood.In the next stage of our research, we compared the correlation of galectin-3 with inflammatory cytokines and hemodynamic indicators of the heart in patients with CHF developed on the basis of rheumatic defects. Galectin-3 with IL-6 (r=0.542, p<001), a-O'NO (r=0.543, p<001), weak positive correlation with S-reactive protein (r=0.259, p<0.004) and antistreptolysin-O (r=0.256, p<0.004) was noted. A weak negative correlation (r=-0.287, p<0.002) was found between left ventricular ejection fraction and IL-6.C-reactive protein with galectin-3 (r=0.577, p<001) and a-TNF(r=0.501, p<001), weak positive correlation was noted between left ventricular myocardial mass (r=0.409, p<0.001) and patients' age (r=0.375, p<0.001). A weak negative (r=-0.301, p<0.001) correlation was observed with the left ventricular ejection fraction.a-with tumor necrosis factor C-reactive protein between (r=0.238, p<009) weakly positive and weakly negative between left ventricular ejection fraction (r=-0.243, p<0.01) a correlational relationship was established. However, a reliable relationship was noted even if the correlation relationship with the left ventricular myocardial mass was not determined. (r=0.190, p<0.05).A weak negative correlation was found between left ventricular myocardial mass and ejection fraction (r=-0.231, p<0.05), and a weak positive correlation with patients' age (r=0.351, p<001).The conducted correlational analysis confirms that long-term inflammation and fibrosis processes in patients with CHF developed on the basis of rheumatic defects have a negative effect on the functional state of the heart independently of hemodynamic disorders. Taking this into account, prescribing a complex standard treatment of CHF containing empagliflozin to this group of patients leads to the stabilization of this severe complication and a significant improvement in the quality of life of patients.Also, at the next stage of our research, we evaluated the effects of galectin-3, which is considered as a fibrosis marker, on other indicators using regression analysis. Table 1 below shows the results obtained
|
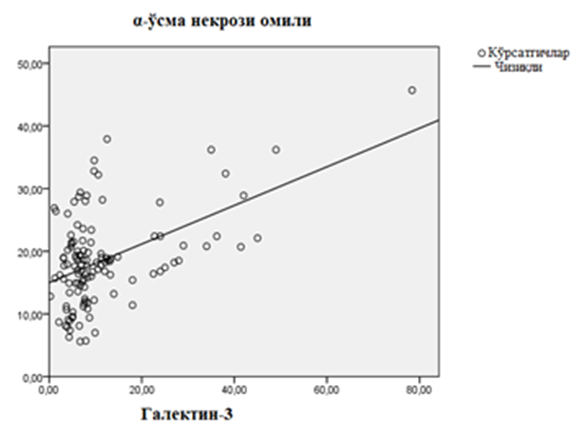 | Figure 1. Linear regression relationship of galectin-3 and α-tumor necrosis factor in patients with chronic heart failure developed on the basis of rheumatic heart defects |
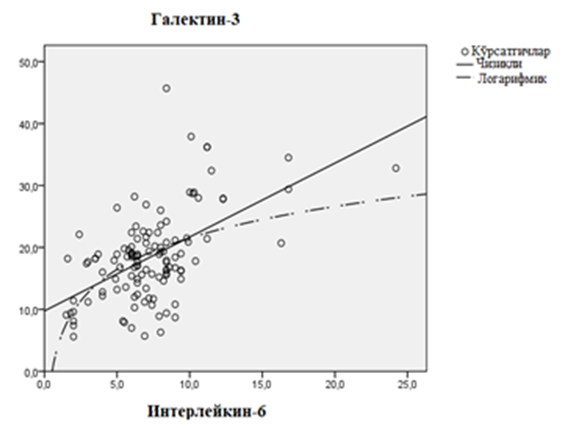 | Figure 2. Linear regression relationship of galectin-3 and interleukin-6 in patients with chronic heart failure developed on the basis of rheumatic defects |
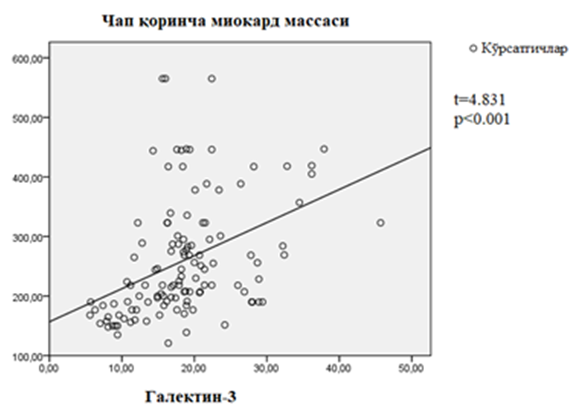 | Figure 3. Correlation between galectin-3 and left ventricular myocardial mass in patients with chronic heart failure developed on the basis of rheumatic defects |
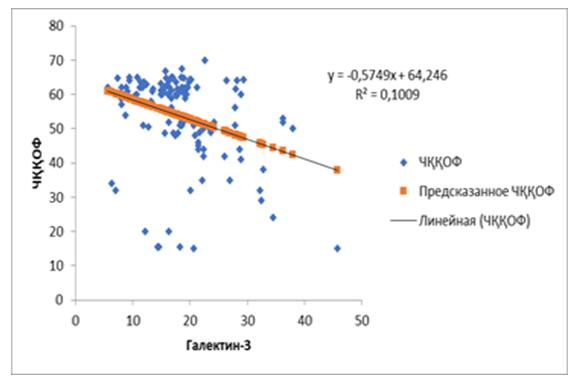 | Figure 4. Correlation between pretreatment galectin-3 and left ventricular ejection fraction in patients with chronic heart failure developed on the basis of rheumatic defects |
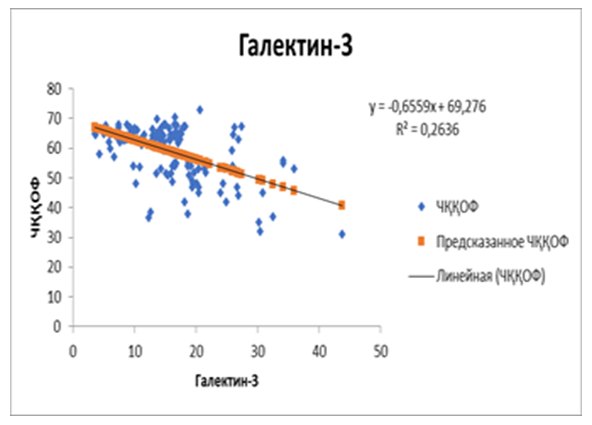 | Figure 5. Correlation between postoperative galectin-3 and left ventricular ejection fraction in patients with chronic heart failure developed on the basis of rheumatic defects |
3. Conclusions
- It was found that the hemodynamic changes observed in SUE developed on the basis of rheumatic heart defects have negative deviations in a number of indicators from the changes in heart failure caused by SCI. This can be attributed to the long and hidden course of inflammatory processes in the first group. As a result, these processes lead to the development of fibrotic processes in the interstitial tissue of the heart, which is confirmed by the high levels of galectin-3 in the blood of the main group of patients. The regression analysis showed that the left ventricular ejection fraction decreased in parallel with the increase in galectin-3 index before the treatment. This confirms that it is possible to predict cardiac functional status from galectin-3 using this assay. In patientsReduction of galectin-3 in the blood after standard treatment with sodium glucose cotransporter type 2 inhibitors led to positive changes in left ventricular ejection fraction. The obtained result showed that empagliflozin in the performed treatments has a positive effect on inflammation and fibrosis processes, and stabilizes hemodynamic processes, not only in ischemic heart disease, but also in CHF developed on the basis of rheumatic defects.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML