-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(4): 869-873
doi:10.5923/j.ajmms.20241404.15
Received: Mar. 9, 2024; Accepted: Mar. 29, 2024; Published: Apr. 2, 2024

The Health-Related Quality of Life of Perimenopausal Women after Coronavirus Infection
Sevara Irgasheva1, Nozima Azamkulova2
1Doctor of Medical Science, Republican Specialized Scientific and Practical Medical Center of Health Mother and Child, Uzbekistan
2Ph.D Student, Republican Specialized Scientific and Practical Medical Center of Health Mother and Child, Uzbekistan
Correspondence to: Sevara Irgasheva, Doctor of Medical Science, Republican Specialized Scientific and Practical Medical Center of Health Mother and Child, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

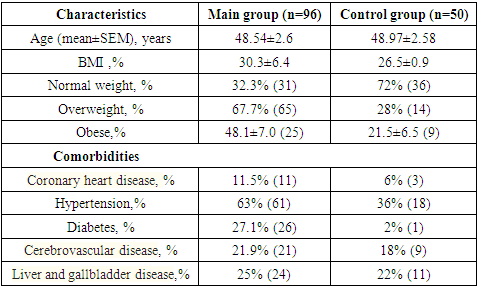
It has been found that women with lower estrogen levels may have more severe course of Covid-19 and its complications. Perimenopausal women have a higher risk of morbidity due to the peculiarities of age-related homeostasis, since estrogen deficiency reduces the functions of varius organs and systems. Perimenopause in some women is accompanied by symptoms of estrogen deficiency and is associated with the onset of MHT. In this regard, research on this issue is in demand. Assessment of the health-related quality of life in perimenopausal women after a coronavirus infection. 146 women aged 45 to 54 years were interviewed and examined. 96 of them were treated 12-16 weeks ago in hospital for COVID-19. The comparison group consisted of 50 women who did not have coronavirus infection. All of them underwent the following assessment: interview, medical examination, blood tests for hormones and biochemical blood tests. Comparative analysis showed, that women who had COVID-19 in perimenopause had more severe symptoms of neurovegetative, psychoemotional and metabolic disorders, and an increase in the number of menstrual irregularities. A general assessment of the state of health is given, characteristic changes in hormonal and biochemical tests are identified. Women who have experienced COVID-19 have more severe perimenopausal disorders, which significantly affects the quality of life.
Keywords: Perimenopause, Menopausal syndrome, COVID-19, Post-menopausal syndrome, Women's health status, Modified Kupperman menopausal index
Cite this paper: Sevara Irgasheva, Nozima Azamkulova, The Health-Related Quality of Life of Perimenopausal Women after Coronavirus Infection, American Journal of Medicine and Medical Sciences, Vol. 14 No. 4, 2024, pp. 869-873. doi: 10.5923/j.ajmms.20241404.15.
1. Introduction
- With the global aging of the population and the emphasis on "active aging" in recent years, increased attention has been directed towards the age-related aspects of women's health. Notably, the incidence of severe outcomes from COVID-19 in women worldwide is considerably lower than in men. This observation suggests the existence of specific protective mechanisms within the female body, potentially contributing to a therapeutic effect that reduces morbidity and mortality from COVID-19. Reports indicate that young women are less prone to severe Covid compared to men, and a reduction in estrogen levels during peri- and postmenopause is linked to an increased risk of severe forms of the disease [1,2,3].Perimenopause typically begins after the age of 45, although individual age variations exist. Clinical studies have demonstrated the pivotal role of estrogens, whose levels decline significantly during this period, in the normal functioning of various organs and systems [5]. The immune system's favorable response to estrogen stimulation is associated with enhanced survival and tolerance of COVID-19 in women under 45.The term "Long COVID" or post-COVID syndrome refers to a collection of symptoms that emerge a month or more after the acute phase of COVID-19. It is now recognized that certain clinical symptoms can persist for more than 3-6 months. In September 2020, the International Classification of Diseases, 10th Revision, introduced a separate code for post-COVID syndrome: "state U09.9 after COVID-19" and U08 (personal history of COVID-19), with code U08.9 recommended for recording "an earlier episode of confirmed or probable COVID-19 that affects the person's health status, and the person no longer has COVID-19". Common post-COVID symptoms include fatigue, muscle and joint pain, decreased physical strength and endurance, and challenges in daily activities. Mental health issues, such as anxiety disorders, depression, and emotional stress, are also prevalent [4,5]. Collectively, these conditions negatively impact both health and various aspects of women's lives. Preserving women's health and quality of life is particularly crucial in such circumstances.Objective: This study aims to assess the health-related quality of life in perimenopausal women following a coronavirus infection.
2. Material and Methods
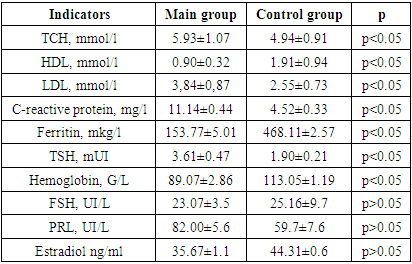
- For the purpose of this study, a total of 146 women within the age range of 45 to 54 years underwent interviews and examinations at the polyclinic of the Republican Scientific and Practical Medical Center of ZMIR and family clinic No. 60. The data collection spanned from January 2022 to December 2023. Among the participants, 96 individuals (designated as the main group) had received treatment for COVID-19 at Zangiata State Specialized Hospital No. 1 and were subsequently invited to participate in the examination. Institutional approval was secured before conducting a retrospective review of their medical records, with the initial examination taking place at least 12 weeks after their hospital stay. The control group comprised 50 women within the same age range who had not experienced a coronavirus infection. Informed voluntary consent was obtained from all participating women. Inclusion criteria for the study: Ø PCR-verified COVID-19 (as per medical documentation) with a confirmed severe or moderate course of the disease.Ø Women within the age range of physiological perimenopause.Ø Willingness to participate in the study, demonstrated by the signing of informed consent.Exclusion criteria: Ø Age under 45 and over 54 years old;Ø Women taking menopausal hormone therapy (MHT) or combined oral contraceptives (COCs) for at least 6 months;Ø Women with premature ovarian failure (POF); cancer currently or in the past.Ø History of previous serotonin syndrome, bipolar personality disorder, uncontrolled seizures, endogenous mental disorders.During the initial phase of the examination, all participants underwent a comprehensive medical history and physical examination. The severity of climacteric syndrome was evaluated using Kupperman's Modified Menopausal Index (MMI), as adapted by E.V. Uvarova. This assessment took into consideration neurovegetative symptoms (such as unstable blood pressure, headaches, vestibulopathy, palpitations, sweating, swelling, increased excitability, drowsiness, sleep disturbance, hot flashes, and asthma attacks), metabolic symptoms, and psycho-emotional symptoms (including fatigue, memory loss, increased tearfulness, changes in appetite, and depression). Each symptom was assigned a score ranging from 1 to 3 points: 0 points for normal, 1 point for mild symptoms, 2 points for moderate severity, and 3 points for pronounced manifestation of menopause. Scoring was conducted separately for each of the three symptom groups, and an overall score was calculated. The values of the Kupperman Modified Menopausal Index (MMI) indicated the severity of climacteric syndrome, with scores of 12-34 points classified as mild, 35-58 points as moderate, and over 58 points as severe [6,7,8]. Additionally, all participants were interviewed using the Greene scale, which encompasses 21 symptom-based questions to assess psycho-emotional states. The questions cover symptoms of depression (1-6 questions), anxiety (7-11 questions), somatic manifestations (12-18 questions), vasomotor symptoms (19-20 questions), and sexual status (21 questions) [9].Laboratory tests: Hormones in the blood serum were assessed through Enzyme-Linked Immunosorbent Assay (ELISA) for the following parameters: follicle-stimulating hormone (FSH), prolactin (PRL), estradiol (E2), and thyroid-stimulating hormone (TSH). Additionally, biochemical blood test indicators were examined, including lipid profile parameters such as total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), and the level of C-reactive protein (CRP), as well as hemoglobin.The monitoring of C-reactive protein is particularly relevant due to its significance as an inflammatory factor in COVID-19, as well as in the post-COVID period [12]. The determination of CRP levels utilized enzyme immunoassay methodology employing a Thermo Scientific Multiscan FC analyzer (China) and the corresponding CRP-ELISA-BEST test system (Vector-Best CJSC, Novosibirsk, Russia).The acquired data underwent statistical processing on a personal computer using the Origin Pro 8.6 program, incorporating a library of statistical functions. The analysis included the calculation of arithmetic mean (M), standard deviation, standard error (m), relative values (frequencies, %), and Student's t-test, with the probability of error (P) also computed.
3. Results
- All 96 women in the main group met the necessary criteria for study participation. Participants in both groups were in the perimenopausal stage and reported complaints characteristic of this life phase. The application of the modified Kupperman scale facilitated the categorization of the considered symptoms into three groups. Coefficients were calculated for each indicator, and their total value was summarized. In the main group, the coefficients for neurovegetative, metabolic, and psychoemotional disorders were statistically significantly higher compared to the control group (refer to Figure 1). For women who experienced a coronavirus infection, the average Modified Menopausal Index (MMI) value was 55.81±2.33, indicating a severe degree of menopausal syndrome. In contrast, the control group exhibited an average MMI value of 33.96±1.13, situated at the borderline between mild and moderate severity.
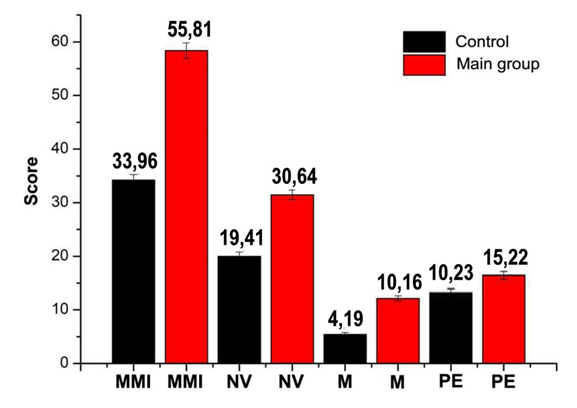 | Figure 1. The results of the Kupperman scale survey in individuals of both clinical groups |
|
|
4. Discussion
- The clinical manifestations of menopausal syndrome are diverse, encompassing symptoms such as hot flashes, sweating, insomnia, lethargy, memory loss, mood instability, and more. It is commonly believed that these symptoms result from functional changes in hypothalamic structures responsible for coordinating temperature, respiratory, cardiovascular reactions, as well as emotional and behavioral responses [13,14]. The neurovegetative, vascular, mental, and endocrine-metabolic disturbances associated with a decrease in estrogen closely resemble the symptoms of post-COVID syndrome [15].The mechanisms underlying the development of nervous system pathology in COVID-19 are linked to neuroinflammation and systemic inflammation [16,17]. It is plausible that the mentioned symptoms share similar pathogenetic mechanisms, representing a response of the central nervous system to stress, whether induced by a virus or a decline in hormone levels. This study found no statistically significant differences in serum estradiol levels between the group of post-COVID women and the group of women without a coronavirus infection. This suggests that more severe perimenopausal disorders may result from hypothalamic dysregulation rather than being directly associated with a decline in ovarian function.It is noteworthy that the risk of thromboembolic complications increases in individuals with prolonged immobilization and the presence of comorbid diseases leading to endothelial dysfunction, such as diabetes mellitus. The unfavorable comorbid background observed in these women may limit the feasibility of using hormonal therapy [18,19].
5. Conclusions
- Perimenopausal women who had moderate and severe forms of COVID -19 have more severe symptoms of neuro-vegetative, psycho-emotional and metabolic disorders compared to women who did not have coronavirus. This notable impact on their quality of life underscores the importance of ongoing observation and the implementation of rational therapeutic interventions. The recommended approaches may include Menopausal Hormone Therapy (MHT) or other alternative methods to address the challenges faced by these women and enhance their overall well-being.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML