-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(3): 760-766
doi:10.5923/j.ajmms.20241403.47
Received: Feb. 29, 2024; Accepted: Mar. 18, 2024; Published: Mar. 29, 2024

A Modern Approach to the Treatment of Chronic Heart Failure with Anemia
Abdigaffar G. Gadaev1, Farida I. Khujakulova2, Nilufar A. Gadaeva1
1Tashkent Medical Academy, Uzbekistan
2Termiz Branch of Tashkent Medical Academy, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In the article, a mutual comparison of the effect of standard medical treatments containing glucose sodium cotransporter type 2 inhibitor dapagliflozin on hematological changes and intracardiac hemodynamics was studied in patients diagnosed with anemia with chronic heart failure. The increase in erythropoietin levels in the group of patients who received dapagliflozin after treatment is related to the effect of the drug on stimulating the production of erythropoietin in the kidney. Also, after the treatment procedures, patients' quality of life was evaluated using the Kansas questionnaire.
Keywords: Chronic heart failure, Dapagliflozin, Kansas survey
Cite this paper: Abdigaffar G. Gadaev, Farida I. Khujakulova, Nilufar A. Gadaeva, A Modern Approach to the Treatment of Chronic Heart Failure with Anemia, American Journal of Medicine and Medical Sciences, Vol. 14 No. 3, 2024, pp. 760-766. doi: 10.5923/j.ajmms.20241403.47.
Article Outline
1. Introduction
- In recent years, the number of patients with chronic heart failure (CHF) has been steadily increasing. On the one hand, this is due to the increase in the average life expectancy of the population in the world, including Uzbekistan, and on the other hand, to the improvement of treatment methods for patients with cardiovascular disease, among which CHF is the leading one. Anemia is one of the most common syndromes in patients with CHF. A large number of clinical studies confirm that 7-79% of anemia occurs in this group of patients [24]. Wide differences in indicators are due to the lack of a uniform approach to the diagnosis of anemia, different causes of the disease and functional classes (FC) of CHF, demographic conditions, and a number of other factors [20]. The Framingham observations were the first to show that anemia is one of the important risk factors of CHF.So far, CHF has not been studied until the end of the pathogenesis of the development of anemia. There are opinions about the influence of hemodilution, renal dysfunction, iatrogenic factors [angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor antagonists (ARA), beta-adrenoblockers (BABs), acetylsalicylic acids], proinflammatory cytokines, malabsorption syndrome, and other conditions [3,29]. Several observations show that the drugs used in the treatment of CHF are of particular importance in the development of erythropoiesis and anemia. It is known that the renin-angiotensin system plays an important role in controlling the number of erythrocytes and the amount of blood plasma [4]. Some data confirm that long-term use of ACEIs in the treatment of CHF leads to anemia. The role of ACEIs in the development of anemia in CHF was shown by A. Ishani and co-authors. According to him, in patients with CHF who had normal hematocrit indicators during the first period of observation, the number of anemia detected after one year of treatment with enalapril reliably increased [13]. In recent years, Glucose-sodium symporter 2 inhibitor drugs, which have many non-glycemic effective hypoglycemic properties, have also been shown to be effective in anemia in patients without CHF diabetes and low left ventricular ejection fraction. In particular, a reliable increase in hematocrit was found in patients taking dapagliflozin belonging to this group. According to some researchers, this situation can be attributed to the increased synthesis of erythropoietin as a result of the nephroprotective effect of the drug [23,21,8]. There are opinions that it has a positive effect on intrarenal hemodynamics, increases natriuresis, reduces the synthesis of pro-inflammatory cytokines, and has a nephroprotective effect [11,7,9].In Uzbekistan, a number of scientific studies have been conducted on the diagnosis, treatment, and the impact of this condition on the quality of life of patients with CHF anemia [30,29,2,12].However so far, the effects of ACEIs and glucose-sodium symporter 2 inhibitors on anemia have not been studied in comparison with CHF with anemia. Taking this into account, we set the following goal.
2. The Purpose of the Study
- A comparative study of hemodynamic, antianemic and nephroprotective effects of angiotensin-converting enzyme inhibitors and glucose-sodium symporter 2 inhibitor dapagliflozin in patients with CHF anemia.
3. Research Materials and Methods
- This scientific research work was conducted in 2021 and 2022 in the cardiology and cardiorehabilitation departments of the multidisciplinary clinic of the Tashkent Medical Academy in patients with CHF developed on the basis of IHD and arterial hypertension. Based on the goals and tasks set before us, the scientific research work was carried out as follows.120 patients with CHF II and III FC were recruited and divided into two groups. The first group was composed of patients with iron deficiency anemia CHF II and III FC who received glucose-sodium cotransporter 2 inhibitor (gliflozins) dapagliflozin as part of complex standard treatment, and the second group included patients with iron deficiency anemia CHF II and III FC who received complex standard treatment without gliflozins organized. Both groups of patients were prescribed iron (III) sucrose intravenously.The first group consisted of 80 patients and their average age was 65.1±1.2 years, 22 (41.5%) men and 31 (58.5%) women. This group, in turn, was divided into two subgroups based on the FC of CHF. The first subgroup consisted of 40 patients with II FS of SYuE, their mean age was 65.2±1.4 years, 24 (60%) men and 16 (40%) women. 26 (65) had myocardial infarction (MI), 11 (27.5) had coronary artery bypass grafting (ACS) or stenting, 8 (20) had obesity, type II diabetes ) - 4 (10%) people.The second subgroup consisted of 40 patients with III FC of CHF. Their average age was 65.1±1.6 years, males were 19 (47.5%) and females were 21 (52.5%). 21 (52.5%) had MI, 9 (22.5%), 9 (22.5%) had ACS or stenting, 11 (27.5%) were obese, 6 (15%) had QD II.The second group consisted of 40 patients, their average age was 66.3±2.0, 20 (50%) men and 20 (50%) women. This group, in turn, was divided into two subgroups based on the FC of CHF. The first subgroup consisted of 20 patients with II FS of CHF, their average age was 68.4±2.1 years, 10 (50%) men and 10 (50%) women. 11 (55%) had MI, 6 (11.3%) had ACS or stenting, 16 (30.1%) were obese, and 4 (7.5%) had QD II.The second subgroup consisted of 20 patients with III FC of CHF. Their average age was 64.4±1.2 years, with 10 (50%) males and 10 (50%) females. There were 17 (85%) who underwent MI, 8 (40%) who underwent ACS or stenting, 16 (30.1%) obesity, 10 (50%) QD type II. The diagnosis of CHF and its FC in patients is based on the complaints of observers, the study of medical history, objective examination and laboratory-instrumental examinations in accordance with the “Recommendations for the diagnosis and treatment of acute and chronic heart failure” updated by the European Association of Cardiology in 2021 and the New York Society of Cardiology (New York It was determined according to the criteria of Heart Association, 1964). In follow-up patients, laboratory-instrumental and functional examinations were performed on 1-3 days after admission to the hospital, and after the first and sixth months of treatment.
4. Analysis and Discussion of Research Results
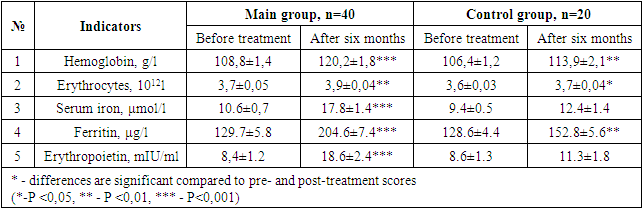
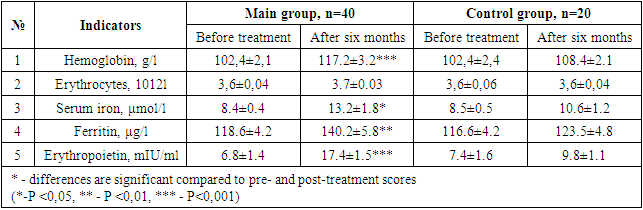
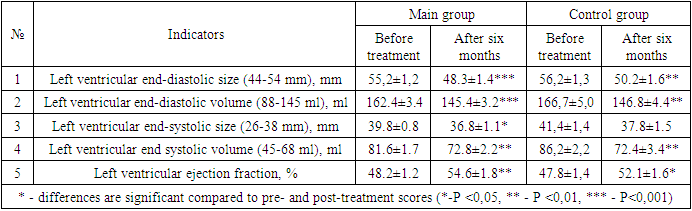
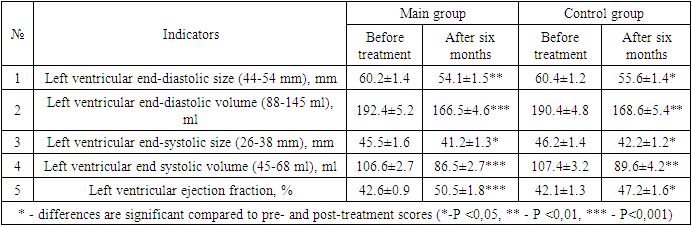
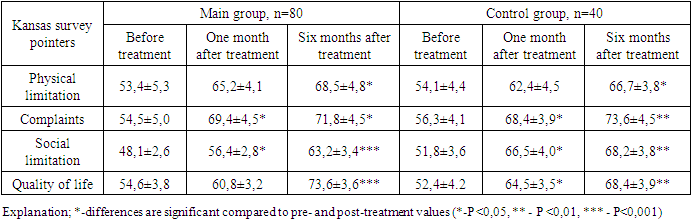
- After the various treatment procedures carried out in our patients, they were monitored dynamically for six months, and all laboratory tests were repeated. Tables 1 and 2 below compare the dynamics of hematological changes before treatment and after six months in patients with CHF II-III FC.
|
|
|
|
|
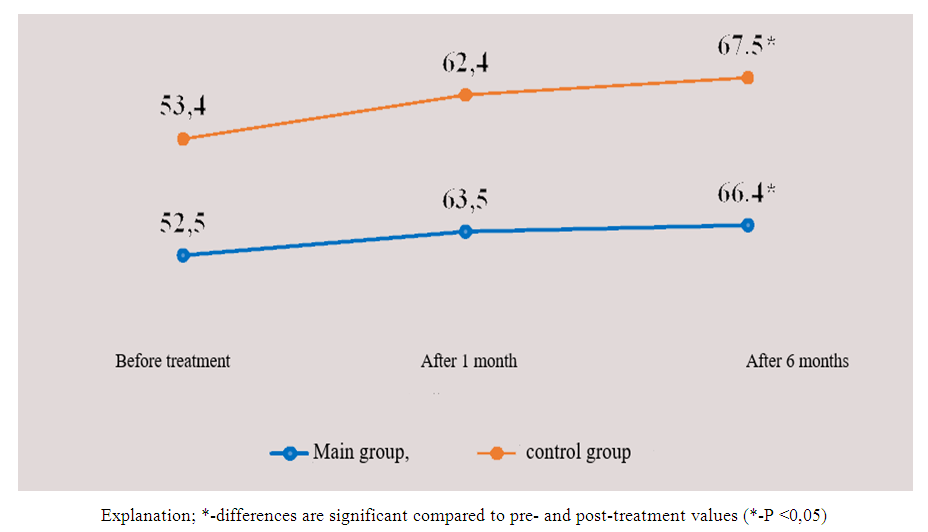 | Figure 1. Changes in the dynamics of the total score of the Kansas questionnaire in patients included in the study |
5. Conclusions
- It was found that the use of dapagliflozin, an inhibitor of glucose sodium cotransporter type 2, as part of standard treatment, has a positive effect on erythropoietin synthesis in patients with chronic heart failure and anemia. It has also been proven that the drug normalizes iron and ferritin levels in the blood, that is, it has an anti-anemic effect. After treatment, dapagliflozin had a positive effect on intracardiac hemodynamic indicators and improved quality of life using the Kansas Questionnaire.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML