-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(3): 734-738
doi:10.5923/j.ajmms.20241403.41
Received: Feb. 25, 2024; Accepted: Mar. 13, 2024; Published: Mar. 22, 2024

Indicators of the Hemostasis System in Women with Premature Birth and Obstetric Blood Loss
Аkhtamova N. A., Shavazi N. N.
Department of Obstetrics and Gynecology 1, Samarkand State Medical University, Samarkand, Uzbekistan
Correspondence to: Аkhtamova N. A., Department of Obstetrics and Gynecology 1, Samarkand State Medical University, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study investigates the hemostasis system in women with premature birth and obstetric blood loss, focusing on the role of endothelial dysfunction in these pregnancy complications. Conducted at the Samarkand State Medical University, the research involved 151 pregnant women aged 18 to 36 years, divided into three groups based on clinical presentations. The study assessed the plasma and cellular components of the hemostasis system, with a particular emphasis on platelets and biochemical markers of endothelial dysfunction, including highly sensitive von Willebrand factor (vWf) and apoptosis protein p53. Results indicated adaptive changes in the hemostatic system during pregnancy, with significant alterations in activated partial thromboplastin time, prothrombin index, fibrinogen levels, and international normalized ratio. Elevated levels of biochemical and functional endothelial markers were associated with the risk of obstetric hemorrhage during preterm labor. The study highlights the importance of monitoring endothelial function as part of prenatal care and suggests that these markers could serve as predictors of complications in pregnant women. Further research is needed to develop effective treatment strategies and improve maternal and child health outcomes.
Keywords: Hemostasis system, Premature birth, Obstetric blood loss, Endothelial dysfunction, Biochemical markers, von Willebrand factor, Apoptosis protein p53
Cite this paper: Аkhtamova N. A., Shavazi N. N., Indicators of the Hemostasis System in Women with Premature Birth and Obstetric Blood Loss, American Journal of Medicine and Medical Sciences, Vol. 14 No. 3, 2024, pp. 734-738. doi: 10.5923/j.ajmms.20241403.41.
1. Introduction
- Obstetric hemorrhage is a leading cause of maternal morbidity and mortality worldwide, despite advancements in obstetric care. Understanding the etiopathogenesis of obstetric hemorrhage and identifying opportunities for improved prognosis, prevention, and treatment are crucial for addressing this persistent issue. The study presented in this article focuses on the hemostasis system in women experiencing premature birth and obstetric blood loss, aiming to shed light on the underlying mechanisms and potential interventions [1-8].The hemostatic system undergoes adaptive changes during pregnancy, playing a vital role in gestation. Dysregulation of this system can lead to complications such as premature birth and obstetric haemorrhage. This research explores the relationship between endothelial dysfunction, a key factor in vascular health, and these pregnancy complications. By examining markers of endothelial function and hemostasis, the study seeks to identify biochemical indicators that could serve as predictors of obstetric hemorrhage and guide the development of effective treatment strategies [4,9-15].Furthermore, the study delves into the impact of various risk factors, including maternal and fetal conditions, obstetric procedures, and socioeconomic factors, on the likelihood of obstetric hemorrhage. Early identification and management of these risk factors through regular prenatal care and appropriate interventions are emphasized as crucial steps in preventing obstetric hemorrhage [16-19].In summary, this research contributes to the growing body of knowledge on the hemostasis system's role in pregnancy and its complications. By investigating the links between endothelial dysfunction, hemostatic changes, and obstetric hemorrhage, the study aims to inform the development of targeted interventions to improve maternal and child health outcomes.
2. Materials and Methods
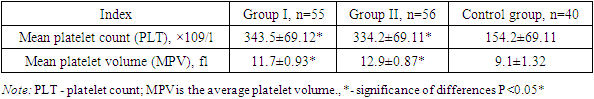
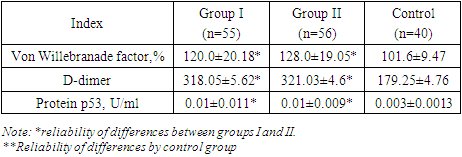
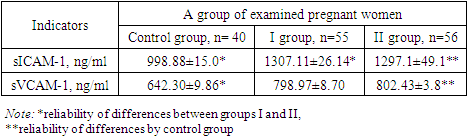
- The study was conducted at the branch of the Russian National Research Medical Center for Medical Research in Samarkand, specifically in the Department of Pathology of Pregnant Women. The research spanned from 2021 to 2023 and involved examining 151 pregnant women, who were subsequently divided into three distinct groups based on their clinical presentations.The inclusion criteria for the study targeted women aged 18 to 36 years, with a particular emphasis on specific age groups. The average age of the participants was 27±2.1 years. The participants' social status varied, with 28% being homemakers and 27% students. The study also assessed the participants' Body Mass Index (BMI), which is relevant as it can influence the volume of blood loss during childbirth.An in-depth analysis of the participants' anamnesis was conducted, encompassing their reproductive, gynecological, somatic, and infectious history. The current pregnancy status, causes of bleeding, and the postpartum period were also assessed. Additionally, the study examined the reproductive and obstetric history of the women, including their parity and gestational age—this comprehensive analysis aimed to identify potential risk factors and correlations with obstetric hemorrhage.The study further delved into the participants' prevalence of somatic diseases and risk factors. The frequency of obesity, myopia, and varicose veins was analysed, as these conditions can potentially impact pregnancy outcomes. Additionally, the assessment covered chronic arterial hypertension (CAH) and iron deficiency anemia (IDA), both of which are significant concerns in prenatal care. The study also considered the presence of chronic pyelonephritis and the incidence of frequent acute respiratory viral infections among pregnant women.The laboratory analyses focused on the hemostasis system, which is crucial for maintaining vascular integrity during pregnancy. The study examined the plasma component of the hemostasis system and the cellular component, with a particular emphasis on platelets. Furthermore, the research aimed to identify biochemical markers of endothelial dysfunction, such as the susceptible von Willebrand factor (vWf) and the apoptosis protein p53 in blood serum. These markers are essential for understanding the functional state of the endothelium and its role in pregnancy complications.The Mann-Whitney test was employed to assess the significance of differences in the studied characteristics for statistical analysis. This non-parametric test is commonly used in medical research to compare two independent groups when the data are not normally distributed. Applying this statistical method allowed for a rigorous evaluation of the data collected in the study, ensuring the validity of the findings.
3. Results
- The analysis was conducted to predict the risk of abnormal blood loss in preterm birth. For this purpose, various features of the anamnesis were studied, including reproductive, gynecological, somatic, and infectious, the course of the current pregnancy, causes of bleeding and the postpartum period.The following results were obtained when analyzing the reproductive history in the compared groups. The average age at menarche was 13.1±1.21 years in the first group, 14.3±1.43 in the second study group and 12.4±1.08 in the control group. As can be seen from the presented data, menarche's age and menstruation duration were statistically comparable in the study groups.A distinctive feature of the obstetric history of pregnant women with UPR is the high frequency of spontaneous abortions in group I (70.7%) in group II (67.6%), threat of premature birth in group I (57.8%); in group II (78.6%), history of premature birth in group I (62.4%); in group II (61.3%).When studying the parity of women in the first group, 12 women (22%) had a 1st birth, 13 women (23.6%) had a 2nd birth, 21 (38.1%) had a 3rd birth, 4 9 women (16.3%) had 1st or more births; in the second group, eight women (14.3%) had 1st births; 11 women (19.6%) had 2nd births. 3rd birth - in 23 (41.1%) 4th or more births - in 14 women (25%) in the control group; 1st birth was in 26 women (65%), 2nd birth - in 9 women (22.5%), 3rd birth - in 4 (10%) 4th - in 1 woman (2.5%) (Fig. 1).
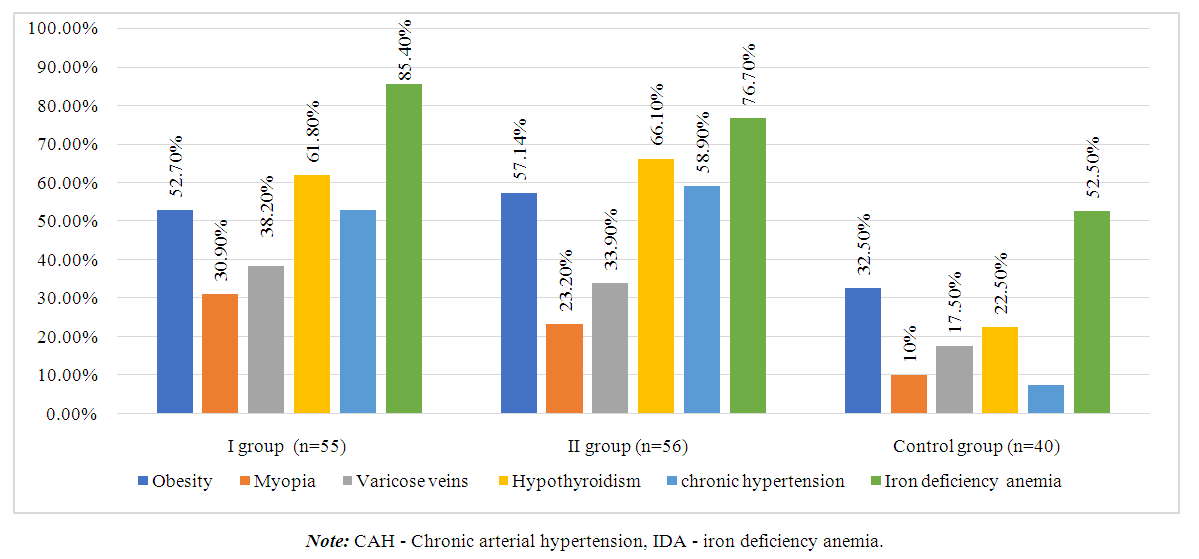 | Figure 1. Analysis of somatic pathology among the study groups |
|
|
|
|
4. Conclusions
- Recently, risk factors for pregnancy loss have traditionally included vascular and hemodynamic disorders in pregnant women, which are observed in various somatic diseases. Generalized endothelial dysfunction is the basis for hemodynamic and microcirculation disorders, including in the uteroplacental basin, developing in various somatic pathologies. There are several hypotheses explaining the development of endothelial dysfunction in UPR.These changes are associated with the intensification of intravascular blood coagulation processes, including in the uteroplacental blood flow. The severity of shifts in the vascular-platelet, coagulation, fibrinolytic and anticoagulant components of hemostasis is determined by the characteristics of the course of pregnancy and the initial state of the coagulation system. Thus, the results of the study show that women with threatened preterm birth have higher values of markers (von Willebrand factor, apoptosis marker p53 protein, D-dimer and levels sICAM-1 and sVCAM-1) than in women with a physiological pregnancy. This confirms the involvement of endothelial dysfunction and apoptosis in the possible development of pathological bleeding during premature birth and the postpartum period.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML