-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(3): 693-697
doi:10.5923/j.ajmms.20241403.33
Received: Feb. 16, 2024; Accepted: Feb. 28, 2024; Published: Mar. 9, 2024

Rehabilitation of Reproductive Function in Women Who have Undergone Termination of Non-Viable Pregnancy Associated with COVID-19
Ruzmetova N. F.1, Shukurov F. I.2, Yuldasheva M. A.3
1Assistant of the Department of Obstetrics and Gynecology Urgench Branch, Tashkent Medical Academy, Tashkent, Uzbekistan
2Doctor of Medical Sciences, Head of the Department of Obstetrics and Gynecology, Tashkent Medical Academy, Tashkent, Uzbekistan
3Assistant of the Department of Obstetrics and Gynecology Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Ruzmetova N. F., Assistant of the Department of Obstetrics and Gynecology Urgench Branch, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The study involved 120 women who had undergone termination of non-viable pregnancies associated with coronavirus infection. The first group consisted of 60 women whose non-viable pregnancy was terminated by vacuum aspiration at 6-8 weeks. The second group consisted of 60 women who underwent medical abortion at 10-12 weeks. The control group included 30 women who terminated their pregnancy due to it being unwanted. All patients underwent clinical-laboratory, hormonal, and hemostasis system examinations before and after rehabilitation therapy. In our treatment for the rehabilitation of reproductive function after termination of non-viable pregnancy in women with COVID-19, a drug comprising 3 mg of drospirenone and 15 mg of ethinyl estradiol monohydrate was used. It was administered in cycles for 3-6 months after the removal of the non-viable pregnancy. The effectiveness of the rehabilitation therapy, based on the normalization of menstrual and reproductive function, was assessed during dynamic observation of the patients over 6-12 months. The use of the drug, based on 3 mg of drospirenone and 15 mg of ethinyl estradiol monohydrate, led to the restoration of menstrual and reproductive functions, reduced the power, and increased the frequency of pregnancy occurrences in 93% of cases, confirming its effectiveness in restoring reproductive function after the termination of non-viable pregnancy associated with COVID-19.
Keywords: COVID-19, Non-viable pregnancy, Vacuum abortion, Medical abortion, Rehabilitation, Reproductive function, Esteretta®
Cite this paper: Ruzmetova N. F., Shukurov F. I., Yuldasheva M. A., Rehabilitation of Reproductive Function in Women Who have Undergone Termination of Non-Viable Pregnancy Associated with COVID-19, American Journal of Medicine and Medical Sciences, Vol. 14 No. 3, 2024, pp. 693-697. doi: 10.5923/j.ajmms.20241403.33.
Article Outline
1. Introduction
- The COVID-19 pandemic has had a significant impact on all aspects of healthcare, including women's reproductive health [1-3]. Infection with the coronavirus during pregnancy increases the risk of its termination, including cases of non-viable pregnancies, which poses a significant issue for women's health [4-6]. Each terminated pregnancy negatively affects the state of the reproductive system, leading to repeated early reproductive losses [7-8]. Moreover, complications arising from COVID-19 may include inflammatory processes affecting the reproductive system, hormonal changes, and psycho-emotional disorders [9-11]. Collectively, these factors can complicate the restoration of reproductive function [12-13]. Given the high prevalence of COVID-19 and its potential risks to pregnancy, there is a need to develop effective methods for rehabilitating reproductive function in women who have undergone termination of pregnancy associated with coronavirus infection. The relevance of rehabilitating reproductive function after termination of pregnancy associated with COVID-19 is undeniable and requires deep and comprehensive research [14-15]. Considering all of the above, the search and development of the most effective methods for rehabilitating women who have undergone termination of pregnancy associated with coronavirus infection remain one of the pressing tasks of modern obstetrics and gynecology. In recent years, various contraceptives have been widely used in rehabilitation for patients after the termination of non-viable pregnancies. According to our data, a drug containing 3 mg of drospirenone and 15 mg of estetrol monohydrate has not been used in the rehabilitation of reproductive function in women who have undergone termination of pregnancy associated with coronavirus infection, hence we decided to use this drug for the first time in comprehensive rehabilitation therapy. Esteretta® is a combined oral contraceptive (COC). It contains estetrol as the estrogen component, a synthetic analog of natural estrogen produced by the human fetus's liver during pregnancy. The progestogen component in Esteretta® is represented by drospirenone, which, in a similar dose combined with ethinylestradiol, is included in other COCs.The aim of the study is to evaluate the effectiveness of a drug containing 3 mg of drospirenone and 15 mg of estetrol monohydrate in rehabilitating reproductive function in women who have undergone termination of pregnancy associated with coronavirus infection.
2. Material and Methods of the Study
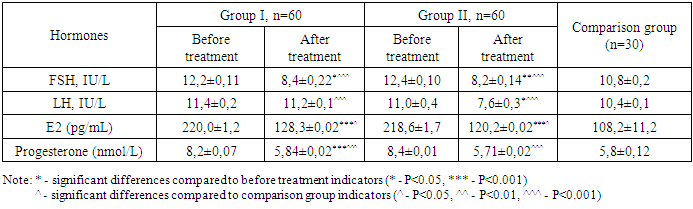
- The study was conducted from 2023 to 2024 at the clinical base of the Department of Obstetrics and Gynecology in the 9th Interdistrict Perinatal Center of Tashkent. All examined patients met the inclusion criteria and did not have diseases related to the exclusion criteria. Inclusion criteria for patients in the study: patients who had undergone termination of non-viable pregnancies associated with coronavirus infection at 6-8 and 10-12 weeks, with informed consent to participate in the study.Exclusion criteria: diabetes mellitus, arterial hypertension, cardiovascular diseases, blood diseases, as well as allergies or intolerance to the components of the drugs used in the study. The study involved 120 women who had undergone termination of non-viable pregnancies associated with coronavirus infection. The first group included 60 women whose non-viable pregnancy was terminated by vacuum aspiration at 6-8 weeks. The second group consisted of 60 women who underwent medical abortion at 10-12 weeks. The control group was made up of 30 women who terminated their pregnancy due to it being unwanted. All patients underwent clinical-laboratory, hormonal, and hemostasis system examinations before and after rehabilitation therapy. Blood hemostasis system analysis was conducted using the automatic coagulometer MINDRAY S3100 (China). Hormone concentration in serum was determined using the chemiluminescence immunoassay Advia Centaur System with Ready Pack kits from Siemens and Beckman Coulter. Hormonal research determined the amount of follicle-stimulating hormone (FSG), luteinizing hormone (LG), estradiol (E2) and progesterone hormones. Blood sampling for hormone concentration determination was performed before and 1-2 months after taking the drug containing 3 mg of drospirenone and 15 mg of estetrol monohydrate. Ultrasound examination was carried out using the Medison Accuvix XQ (Korea) device, utilizing transabdominal and transvaginal convex probes with a frequency of 3.5-5 MHz. After the termination of non-viable pregnancies, each patient was prescribed comprehensive rehabilitation therapy, including antibacterial, immunomodulatory, and hormonal therapy. The standard antibacterial therapy included the administration of doxycycline 100 mg twice a day for 5 days.In our study, a drug containing 3 mg of drospirenone and 15 mg of estetrol monohydrate was used to rehabilitate reproductive function after the termination of non-viable pregnancies in women with COVID-19. Contraceptive intake began on the first day after the termination of the non-viable pregnancy and continued for 3-6 months. When prescribing the drug, family thrombotic history was also assessed: the presence of thrombosis and thromboembolisms in relatives, heart attacks, strokes, habitual miscarriages, etc. During the hemostasiogram, the following blood coagulation parameters were studied: activated partial thromboplastin time (APTT), D-dimer, platelets, fibrinogen, thrombin time, and prothrombin index (PTI). The effectiveness of the rehabilitation therapy was assessed based on the normalization of menstrual and reproductive function during dynamic observation of the patients over 6-12 months.Statistical data processing was carried out using the Statistica v.6.0 application package and Microsoft Excel 2000 software. Results were processed using methods of variational statistics and are presented as M±m. The assessment of the significance of differences in mean values and relative indicators was carried out using the t-test (Student's t-test). A significance level of P<0.05 was adopted for the study.Ethical Aspects. The study procedure doesn't interfere with any ethical basics and oral agreement was taken to all patients.
3. Results
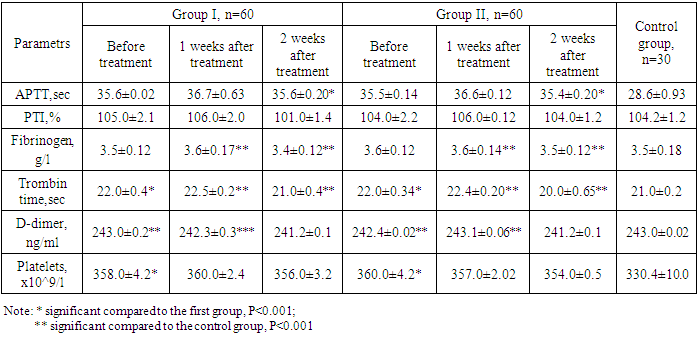
- The age of the women ranged from 18 to 36 years (average 28.4±0.24). Primigravidas accounted for 41.3%, and multigravidas 58.7% of the total number of women included in the study. Most frequently, women reported complaints of dull pains in the lower abdomen and bloody discharges from the genital tract – 47.3% of them reported pain only – 19.2%; bloody discharges only – 28.1% of women.Considering patients with COVID-19 are at high risk of thromboembolic complications, we conducted a study of the hemostasiogram.Analysis of hemostasiograms in patients was carried out before and after 1-2 and 4 weeks of taking the drug containing 3 mg of drospirenone and 15 mg of estetrol monohydrate. The results of the study of the hemostasis system after 1 week in women taking 3 mg of drospirenone and 15 mg of estetrol monohydrate showed an extension of the activated partial thromboplastin time (APTT), which was 36.7±0.63 sec and 36.6±0.12 sec, respectively. In the control group, this time was 28.6±0.93 sec (p<0.001).The level of D-dimer in the groups was respectively 242.3±0.3 ng/ml and 243.1±0.06 ng/ml, in the control group - 243.0±0.02 ng/ml. The study of fibrinogen level showed a slight increase in its concentration, which was 3.6±0.17 g/l and 3.6±0.14 g/l in the groups, and in the control group - 3.5±0.18 g/l (p<0.001). Analysis of the prothrombin index (PTI) in the groups showed respectively 106.0±2.0% and 106.0±0.12% (p<0.001). The study of thrombin time also showed a slight increase in its duration and was respectively 22.5±0.2 sec and 22.4±0.20 sec in the groups (p<0.001) (see table 1).
|
4. Discussion
- The discussion of the findings from this study sheds light on several important aspects regarding the rehabilitation of reproductive function in women after the termination of pregnancy associated with COVID-19, using a drug containing 3 mg of drospirenone and 15 mg of estetrol monohydrate. The significance of these findings can be understood in the broader context of obstetrics and gynecology, especially considering the unique challenges presented by the COVID-19 pandemic.The results indicate that the drug containing 3 mg of drospirenone and 15 mg of estetrol monohydrate is effective in rehabilitating reproductive function, as evidenced by the restoration of menstrual function in a significant majority of the study participants and the subsequent high pregnancy rates. This suggests that the drug regimen not only aids in the physical restoration of the reproductive system but also ensures its functional recovery, which is crucial for women desiring future pregnancies.The study provides valuable insights into the drug's impact on hormonal levels and the hemostasis system, which are critical factors in the context of reproductive health. The minimal impact on the hemostasis system is particularly noteworthy, given the increased thrombotic risk associated with COVID-19. This aspect underscores the safety profile of the drug regimen, making it a viable option for women recovering from COVID-19-related reproductive challenges.The comparison with control groups provides a robust framework for evaluating the drug's efficacy. The significant differences observed in hormonal levels, menstrual function restoration, and pregnancy rates as compared to control groups highlight the drug's specific benefits in the targeted population. This comparative analysis reinforces the argument for the drug's role in targeted rehabilitation efforts.The findings have significant implications for clinical practice, particularly in the formulation of guidelines for managing reproductive health issues in the aftermath of COVID-19. The demonstrated efficacy and safety profile of the drug regimen suggest that it could be recommended for widespread use in similar cases, providing healthcare professionals with an evidence-based approach to addressing these challenges.In conclusion, the discussion of these findings emphasizes the potential of this drug regimen in addressing a critical aspect of women's health affected by the COVID-19 pandemic. It offers a promising therapeutic option for rehabilitating reproductive function in women impacted by COVID-19-associated pregnancy terminations, with a strong emphasis on safety, efficacy, and clinical applicability. The study's contributions to the field of obstetrics and gynecology are significant, providing a foundation for future research and clinical practice enhancements.
5. Conclusions
- The use of a drug containing 3 mg of drospirenone and 15 mg of estetrol monohydrate in rehabilitation is a highly effective method for restoring reproductive function in patients after the termination of a non-developing pregnancy associated with COVID-19. Rehabilitation therapy using a drug containing 3 mg of drospirenone and 15 mg of estetrol monohydrate allows for the restoration of menstrual and reproductive functions, reduces the frequency of complications, and increases the likelihood of pregnancy in 93% of cases, confirming its high efficacy in restoring reproductive function after the termination of a non-developing pregnancy associated with COVID-19. Its minimal impact on the hemostasis system, favorable hormonal effects, and significant success in restoring menstrual function and fertility, all without significant side effects, make it a promising therapeutic choice.
Conflict of Interests
- The authors declare no conflict of interest.
References
| [1] | Adamyan, L.V., Aznaurova, Y.B., & Filippov, O.S. (2020). COVID-19 and women's health (Literature review). Problems of Reproduction, 2, 6-17. |
| [2] | Allotey, J., Stallings, E., Bonet, M., et al. (2020). Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ, 370, m3320. https://doi.org/10.1136/bmj.m3320. |
| [3] | Breslin, N., Baptiste, C., Miller, R., Fuchs, K., Goffman, D., Gyamfi-Bannerman, C., & D'Alton, M. (2020). Coronavirus disease 2019 in pregnancy: Early lessons. American Journal of Obstetrics & Gynecology MFM, 2(2), 100111. https://doi.org/10.1016/j.ajogmf.2020.100111. |
| [4] | Temporary Clinical Guidelines for Managing COVID-19 During Pregnancy, Childbirth, and the Postpartum Period. (2020). Tashkent, p. 31. |
| [5] | Shchegolev, A.I., Kulikova, G.V., Tumanova, U.N., et al. (2021). Morphometric characteristics of placental villi in women with COVID-19. Bulletin of Experimental Biology and Medicine, 172 (7), 102-107. https://doi.org/10.1007/s10517-021-05109-0. |
| [6] | Radzinsky, V.S. (2015). Non-developing pregnancy. Methodical recommendations by MARS (Interdisciplinary Association of Reproductive Medicine Specialists). Moscow: Status Praesens Journal Editorial, p. 48. |
| [7] | Kholova, Z.B., & Shukurov, F.I. (2023). Morphological features of fetoplacental dysfunction in pregnant women with COVID-19. In Proceedings of the XVII International Congress on Reproductive Medicine (pp. 133-134). Moscow. |
| [8] | Ruzmetova, N.F., & Shukurov, F.I. (2023). Assessment of clinical and diagnostic criteria for non-developing pregnancy in women with COVID-19. In Proceedings of the XVII International Congress on Reproductive Medicine (pp. 80-81). Moscow. |
| [9] | Mamazhanova, D.M., & Shukurov, F.I. (2023). The state of hormonal status in pregnant women vaccinated against COVID-19. In Proceedings of the XVII International Congress on Reproductive Medicine (pp. 115-116). Moscow. |
| [10] | Kholova, Z.B., Kholmatova, D.A., & Shukurov, F.I. (2022). A new approach to the treatment and prevention of fetoplacental dysfunction in pregnant women infected with COVID-19. Journal of Dermatovenereology and Reproductive Health News, 3-4 [99-100], 38-39. http://repository.tma.uz/xmlui/handle/1/4489. |
| [11] | Mamazhanova, D.M., & Shukurov, F.I. (2022). Characteristics of the hemostasis system in pregnant women vaccinated against COVID-19. New Day in Medicine, 10(48), 47-51. http://repository.tma.uz/xmlui/handle/1/4469. |
| [12] | Mamazhanova, D.M., & Shukurov, F.I. (2022). Vaccinating pregnant women against COVID-19: Safety, efficacy, assessment and prediction of post-vaccination immunogenicity levels. Tashkent: Methodological Recommendations, p. 40. http://repository.tma.uz/xmlui/handle/1/2699. |
| [13] | Rasmussen, S.A., Smulian, J.C., Lednicky, J.A., Wen, T.S., & Jamieson, D.J. (2020). Coronavirus disease 2019 (COVID-19) and pregnancy: What obstetricians need to know. American Journal of Obstetrics and Gynecology, 222(5), 415-426. https://doi.org/10.1016/j.ajog.2020.02.017. |
| [14] | Craig, A.M., Hughes, B.L., & Swamy, G.K. (2021). Coronavirus disease 2019 vaccines in pregnancy. American Journal of Obstetrics and Gynecology MFM, 3(2), 100295. https://doi.org/10.1016/j.ajogmf.2020.100295. |
| [15] | Chen, H., Guo, J., Wang, C., et al. (2020). Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet, 395, 809-815. https://doi.org/10.1016/S0140-6736(20)30360-3. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML