-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(3): 635-639
doi:10.5923/j.ajmms.20241403.20
Received: Feb. 13, 2024; Accepted: Mar. 4, 2024; Published: Mar. 6, 2024

Associations between IL1b (Rs1143627) Polymorphisms and Susceptibility to Uterine Atony
Umida A. Ashurova1, Dilbar K. Najmutdinova2, Lagiya M. Abdullaeva3
1Post-Doctoral Researcher, Department of Obstetrics and Gynecology in Family Medicine, Tashkent Medical Academy, Tashkent, Uzbekistan
2Head of Department of Obstetrics and Gynecology in Family Medicine, Tashkent Medical Academy, Tashkent, Uzbekistan
3Department of Obstetrics and Gynecology of Postgraduate Faculty, Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Umida A. Ashurova, Post-Doctoral Researcher, Department of Obstetrics and Gynecology in Family Medicine, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Postpartum hemorrhage affects 3–10% of deliveries and accounts for nearly 20% of maternal deaths worldwide. This study was aimed to find out genetic predisposition for postpartum hemorrhage in order to develop risk groups and preventive measures. Thus, polymorphism in IL1B gene were investigated. There was not found association with T-31C polymorphism in ILIB gene and risk of postpartum uterine atony among Uzbek women.
Keywords: Postpartum hemorrhage, Uterine atony, T-31C polymorphism in IL1B gene, Genetic mutation
Cite this paper: Umida A. Ashurova, Dilbar K. Najmutdinova, Lagiya M. Abdullaeva, Associations between IL1b (Rs1143627) Polymorphisms and Susceptibility to Uterine Atony, American Journal of Medicine and Medical Sciences, Vol. 14 No. 3, 2024, pp. 635-639. doi: 10.5923/j.ajmms.20241403.20.
1. Introduction
- Postpartum hemorrhage affects between 3% and 10% of all births and accounts for approximately 20% of all maternal deaths worldwide [1,2]. The leading cause of this condition is uterine atony, which is responsible for about 70% of these incidents [3]. Early detection of individuals at risk, along with proactive planning and improved monitoring, can significantly reduce the health risks and fatalities associated with postpartum hemorrhage [1,4].Interleukin-1 (IL1), a major pro-inflammatory cytokine, plays a crucial role in the inflammatory process related to this condition [5,6]. Initially identified as a protein that induces fever, IL-1 has been recognized as a critical factor in the development of various inflammatory diseases. Among the IL-1 family, IL-1β stands out as a powerful inflammatory cytokine linked to the progression of autoinflammatory diseases [7,8].The activation and release of IL-1β involve a complex process that includes the inflammasome, a multi-protein complex inside cells. The inflammasome's activation triggers the conversion of pro-IL-1β into its active form by caspase enzymes, a reaction that can be initiated by various signals such as pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). When activated, IL-1β attaches to its receptor (IL-1R) on adjacent cells, leading to a series of inflammatory reactions that include attracting neutrophils and other immune cells to the site.The IL1 family includes IL1A, IL1B, and an antagonistic cytokine known as the IL1 receptor antagonist (IL1RA) [5,6]. IL1A and IL1B serve as inflammatory agents, produced by various cell types in response to different triggers. These cytokines impact endothelial cells by promoting the expression of adhesion molecules and contributing to prothrombotic states. Conversely, IL1RA, which occurs naturally, acts as a competitive inhibitor, potentially dampening the immune response [9,10]. The genes for IL-1 cytokines (IL-1A, IL-1B, and IL-1RN) are situated within a 430 kb segment on chromosome 2q12-21, with the IL-1β gene located specifically at chromosome 2q13 [11], featuring 7 exons and 6 introns. Research on gene polymorphisms has primarily concentrated on the positions −511 bp, −31 bp, and +3945 bp from the IL-1β gene's transcription start site, which are known to undergo C-T base pair mutations [12,13]. Studies have frequently examined polymorphisms within the IL1 gene cluster, including IL1B and IL1RN (which encodes IL1RA), revealing associations with the plasma levels of IL1B and ILRA [14,15].Consequently, the purpose of this research was to assess the association between the T-31C polymorphism in the IL1B gene and the risk of postpartum hemorrhage among Uzbek women.
2. Material and Methods
- We conducted a study involving 101 women diagnosed with postpartum uterine atonic hemorrhage of varying severity, who comprised the main group. The diagnosis of postpartum uterine atonic hemorrhage was made according to the criteria of the national clinical protocol of the Republic of Uzbekistan, "Prevention and Management Tactics for Postpartum Obstetric Hemorrhages," approved on March 1, 2021. According to the protocol, the diagnosis of postpartum uterine atonic hemorrhage was established when: blood loss was ≥ 500 ml during childbirth through natural delivery; blood loss was ≥ 1000 ml during a cesarean section (CS) operation; and any clinically significant volume of blood loss (leading to hemodynamic instability) occurred within 12 weeks after childbirth. Exclusion criteria included women whose uterine atonic hemorrhage developed due to retained placental tissues or membranes, birth canal trauma, and coagulation disorders unrelated to bleeding. The control group consisted of 103 women without significant chronic somatic pathology who had a history of uncomplicated natural deliveries without obstetric complications. All the studied women were of Uzbek nationality. Written informed consent was obtained from all patients for their participation in the study.All women underwent clinical, laboratory, and instrumental investigations, including standard methods for collecting medical history and physical examinations.Molecular-genetic research was conducted at the Department of Molecular Medicine and Cell Technologies of the Republican specialized scientific and practical medical center of Hematology of the Republic of Uzbekistan.The research utilized whole venous blood to investigate polymorphism. To identify the T-31C polymorphism in the IL1B gene at the molecular-genetic level, preparations of genomic DNA were employed. These DNA molecules were isolated from peripheral venous blood through the use of the "DNA-Extran-1" kit, provided by “Synthol”, Russia. Polymorphism detection was performed on instruments of "Applied Biosystems" 2720 (USA) and RT PCR Rotor-Gene 6000 (Corbett Research, Australia), using a kit of LLC "Litekh" (Moscow), following the manufacturers' instructions. PCR products were separated by electrophoresis in a 2% agarose gel.The statistical analysis was performed using the software package "STATISTICA 10.0". In the statistical processing of clinical material, mathematical statistical methods were employed, including: - Frequency analysis (%); - The study employed various statistical methods including the calculation of the mean (M), standard deviation (σ), standard error (m), and others. Additionally, it used an analysis of variance through the t-test, and correlation analysis utilizing Pearson's correlation coefficient (r). The statistical significance of differences in qualitative characteristics was determined using Fisher's exact test and Pearson's χ2 criterion. A significance level threshold was established at 0.05. The distribution of genotypes was checked for Hardy-Weinberg equilibrium using the computer program "GenePop" (http://wbiomed.curtin.edu.au/genepop) and evaluated using the χ2 criterion. For the analysis of the association of polymorphisms in the studied genes, frequency analysis methods ROC, AUC, OR with a 95% confidence interval (95% CI) were also utilized.
3. Results
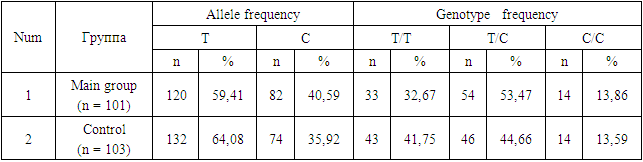
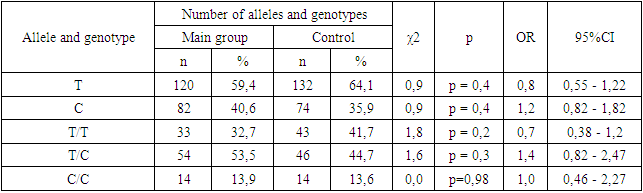
- The age of the patients did not show significant differences. When examining obstetric indicators, in the main group of women who later developed postpartum uterine atony, multiparous women predominated by parity, accounting for 61.4%, compared to primiparous women, which constituted 38.6% of cases. Such predominance may be associated with changes in the receptor apparatus of the myometrium and the contractile ability of the muscle tissue as the number of deliveries increases. Complicated pregnancies in the main group were observed in 27.7% of patients, and apparently, a complicated gestation does not have such a significant impact on the development of postpartum hemorrhage. As for the gestational age, preterm births were noted in 32.7% of women at 29-36 weeks of pregnancy.In the studied groups of patients and the control group, the observed distribution of genotype frequencies for this polymorphism did not deviate from Hardy-Weinberg equilibrium, i.e., it corresponded to the calculated expected values (χ² < 3.4; p > 0.05). Considering the sufficient sample size of patients and controls (101 and 103, respectively), it can be concluded that there are no deviations from Hardy-Weinberg equilibrium. The significant compliance with Hardy-Weinberg equilibrium indicates the homogeneity of the studied groups of patients with postpartum uterine atony and the control group.The distribution of genotype and allele frequencies for the T-31C polymorphism in the IL1B gene is presented in Table 1. It was established that in the study sample, the dominant T allele was found in 59.41% of cases, while the recessive C allele was found in 40.59% of cases. The mutant CC genotype in the patient group was registered in 13.86% of cases, the heterozygous TC genotype in 53.47% of cases, and the dominant TT genotype in 32.67% of cases, with control group figures at 41.75%, 44.66%, and 13.59% of cases, respectively (Table 1).
|
|
4. Discussion
- Current research on gene polymorphisms primarily targets specific base positions at −511 bp, −31 bp, and +3945 bp from the start of the IL-1β gene transcription, all of which are characterized by C-T base mutations [12,13]. These polymorphisms, particularly at the −511 (rs16944) locus in the IL-1β promoter region, have been linked to increased expression of IL-1β, intensifying the inflammatory response [16]. In a study conducted by Tripathi et al., PCR-RFLP techniques were utilized in a case-control investigation involving 200 patients with type 2 diabetes mellitus (T2DM) and 223 healthy individuals in northern India. This study uncovered a significant association between the IL-1β −511 C/T gene polymorphism and T2DM, indicating that carriers of the T allele are at a heightened risk of developing T2DM [17]. Similarly, Tayel et al. employed real-time fluorescence quantitative PCR (qPCR) to analyze the IL-1β −511 T > C (rs16944) single-nucleotide polymorphisms (SNPs) in 50 T2DM patients and 30 healthy controls, discovering that those with the CC genotype exhibited the highest levels of IL-1β transcripts (P < .001), suggesting an increased risk of diabetes associated with the rs16944 polymorphism in the IL-1β gene [18].Additional findings suggest that the IL1B-511AA genotype could raise the risk of septic shock and its mortality in adults [19]. Meanwhile, research into the relationship between the IL1B gene and preterm birth (PTB) demonstrates inconsistent outcomes across various populations. Schmid and colleagues observed a significant link between the IL1B rs1143634 (+3953C/T) polymorphism and a lower risk of PTB within the Austrian population. On the other hand, Edwards and colleagues did not find a significant association between the IL1B rs1143634 (+3953C/T) polymorphism and PTB in the U.S. population [20], underscoring the disparate findings of genetic association studies on the IL1B gene and PTB across different demographics.In research conducted by Jianting M. and others, a notable association was discovered between the IL1B -511 T > C polymorphism and a heightened risk of recurrent miscarriage. Conversely, the IL6 -634C > G polymorphism was significantly linked to a reduced risk of recurrent miscarriage [21]. These findings further illustrate the complex interplay between genetic polymorphisms and reproductive health outcomes, emphasizing the importance of considering genetic background in understanding susceptibility to various reproductive complications.In our study, the T-31C polymorphism in the IL1B gene did not have a significant impact on the risk of developing uterine atony in the postpartum period. Carriers of the heterozygous TC genotype show a tendency towards developing atonic PPH, which was not statistically significant. Studies of the T-31C polymorphism in the IL1B gene have been conducted in various pathologies, but this is the first time we have conducted it on the example of PPH in Uzbek women. This research is essential for devising relevant preventative strategies aimed at lowering maternal mortality and morbidity.Thus, it is essential to pay attention to the significance of individual genotypes of selected cytokine gene polymorphisms, as they play a crucial role in various biological processes in the body. Nevertheless, several questions warrant further consideration and exploration. Our study aimed to inspire researchers to conduct further investigations that may hold clinical significance for the diagnosis and prevention of obstetric and gynecological pathologies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML